Page 269 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 269
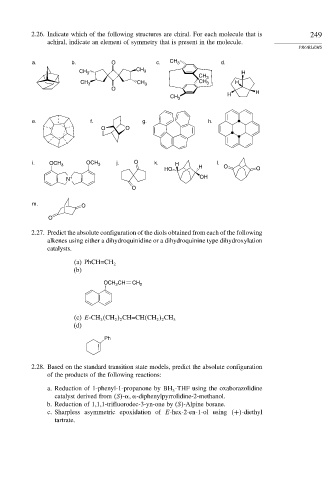
2.26. Indicate which of the following structures are chiral. For each molecule that is 249
achiral, indicate an element of symmetry that is present in the molecule.
PROBLEMS
a. b. O c. CH 3 d.
CH 3 CH 3 H
CH 3
CH 3 CH 3 CH 3 H
O
H H
CH 3
e. f. g. h.
O O
i. OCH 3 OCH 3 j. O k. H l.
HO H O O
N + OH
O
m. O
O
2.27. Predict the absolute configuration of the diols obtained from each of the following
alkenes using either a dihydroquinidine or a dihydroquinine type dihydroxylation
catalysts.
(a) PhCH=CH 2
(b)
OCH CH CH 2
2
(c) E-CH CH CH=CH CH CH
3 2 2 2 2 3
(d)
Ph
2.28. Based on the standard transition state models, predict the absolute configuration
of the products of the following reactions:
a. Reduction of 1-phenyl-1-propanone by BH -THF using the oxaborazolidine
3
catalyst derived from S - -diphenylpyrrolidine-2-methanol.
b. Reduction of 1,1,1-trifluorodec-3-yn-one by (S)-Alpine borane.
c. Sharpless asymmetric epoxidation of E-hex-2-en-1-ol using + -diethyl
tartrate.