Page 263 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 263
2.11. 1,2-Diphenyl-1-propanol can be prepared by hydride reduction of 1,2-diphenyl- 243
1-propanone or by addition of phenylmagnesium bromide to 2-phenylpropanal.
PROBLEMS
Predict the stereochemistry of the major product in each case.
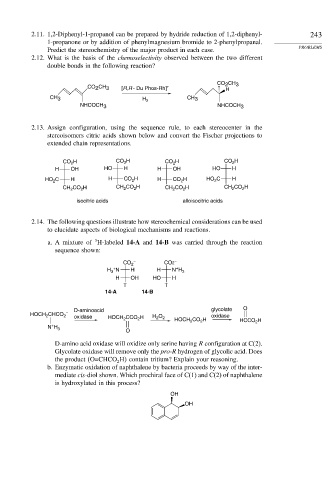
2.12. What is the basis of the chemoselectivity observed between the two different
double bonds in the following reaction?
CO CH 3
2
CO CH 3 [R,R - Du Phos-Rh] + H
2
CH 3 H 2 CH 3
NHCOCH 3 NHCOCH 3
2.13. Assign configuration, using the sequence rule, to each stereocenter in the
stereoisomers citric acids shown below and convert the Fischer projections to
extended chain representations.
CO H CO H CO H CO H
2
2
2
2
H OH HO H H OH HO H
HO C H H CO H H CO H HO C H
2
2
2
2
CH CO H CH CO H CH CO H CH CO H
2
2
2
2
2
2
2
2
isocitric acids alloisocitric acids
2.14. The following questions illustrate how stereochemical considerations can be used
to elucidate aspects of biological mechanisms and reactions.
3
a. A mixture of H-labeled 14-A and 14-B was carried through the reaction
sequence shown:
CO 2 – CO2 –
+
+
H N H H N H 3
3
H OH HO H
T T
14-A 14-B
D-aminoacid glycolate O
CHCO –
HOCH 2 2 oxidase HOCH CCO H H 2 O 2 HOCH CO H oxidase HCCO H
2
2
+
N H 3 2 2 2
O
D-amino acid oxidase will oxidize only serine having R configuration at C(2).
Glycolate oxidase will remove only the pro-R hydrogen of glycolic acid. Does
the product O=CHCO H contain tritium? Explain your reasoning.
2
b. Enzymatic oxidation of naphthalene by bacteria proceeds by way of the inter-
mediate cis-diol shown. Which prochiral face of C(1) and C(2) of naphthalene
is hydroxylated in this process?
OH
OH