Page 260 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 260
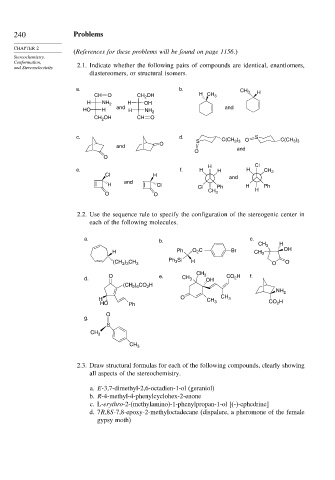
240 Problems
CHAPTER 2
(References for these problems will be found on page 1156.)
Stereochemistry,
Conformation,
and Stereoselectivity 2.1. Indicate whether the following pairs of compounds are identical, enantiomers,
diastereomers, or structural isomers.
a. b. CH
CH O CH OH H CH 3 3 H
2
H NH 2 H OH
HO H and H NH 2 and
CH OH CH O
2
c. d. S
)
S C(CH 3 3 O C(CH 3 ) 3
O
and
O and
O
H Cl
e. f. H H H CH 3
Cl H
and
and
H Cl H Ph
Cl Ph H
CH 3
O O
2.2. Use the sequence rule to specify the configuration of the stereogenic center in
each of the following molecules.
a. b. c.
CH 3 H
H Ph O 2 C Br CH 3 OH
Ph Si
(CH ) CH 3 3 H O O
2 3
CH
O e. 3 CO H f.
d. CH 3 OH 2
(CH ) CO H
2 6
2
NH 2
H O CH CH 3
HO Ph 3 CO H
2
O
g.
S
CH 3
CH 3
2.3. Draw structural formulas for each of the following compounds, clearly showing
all aspects of the stereochemistry.
a. E-3,7-dimethyl-2,6-octadien-1-ol (geraniol)
b. R-4-methyl-4-phenylcyclohex-2-enone
c. L-erythro-2-(methylamino)-1-phenylpropan-1-ol [(-)-ephedrine]
d. 7R,8S-7,8-epoxy-2-methyloctadecane (dispalure, a pheromone of the female
gypsy moth)