Page 258 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 258
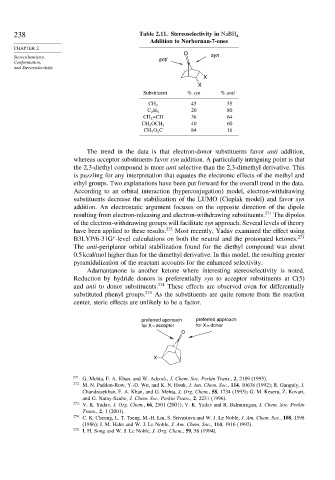
238 Table 2.11. Stereoselectivity in NaBH 4
Addition to Norbornan-7-ones
CHAPTER 2
O syn
Stereochemistry, anti
Conformation,
and Stereoselectivity
X
X
Substituent % syn % anti
45 55
CH 3
20 80
C 2 H 5
CH 2 =CH 36 64
40 60
CH 3 OCH 2
CH 3 O 2 C 84 16
The trend in the data is that electron-donor substituents favor anti addition,
whereas acceptor substituents favor syn addition. A particularly intriguing point is that
the 2,3-diethyl compound is more anti selective than the 2,3-dimethyl derivative. This
is puzzling for any interpretation that equates the electronic effects of the methyl and
ethyl groups. Two explanations have been put forward for the overall trend in the data.
According to an orbital interaction (hyperconjugation) model, electron-withdrawing
substituents decrease the stabilization of the LUMO (Cieplak model) and favor syn
addition. An electrostatic argument focuses on the opposite direction of the dipole
resulting from electron-releasing and electron-withdrawing substituents. 271 The dipoles
of the electron-withdrawing groups will facilitate syn approach. Several levels of theory
have been applied to these results. 272 Most recently, Yadav examined the effect using
B3LYP/6-31G -level calculations on both the neutral and the protonated ketones. 273
∗
The anti-periplanar orbital stabilization found for the diethyl compound was about
0.5 kcal/mol higher than for the dimethyl derivative. In this model, the resulting greater
pyramidalization of the reactant accounts for the enhanced selectivity.
Adamantanone is another ketone where interesting stereoselectivity is noted.
Reduction by hydride donors is preferentially syn to acceptor substituents at C(5)
and anti to donor substituents. 274 These effects are observed even for differentially
substituted phenyl groups. 275 As the substituents are quite remote from the reaction
center, steric effects are unlikely to be a factor.
preferred approach preferred approach
for X = acceptor for X = donor
O
X
271 G. Mehta, F. A. Khan, and W. Adcock, J. Chem. Soc. Perkin Trans., 2, 2189 (1995).
272
M. N. Paddon-Row, Y.-D. Wu, and K. N. Houk, J. Am. Chem. Soc., 114, 10638 (1992); R. Ganguly, J.
Chandrasekhan, F. A. Khan, and G. Mehta, J. Org. Chem., 58, 1734 (1993); G. M. Keseru, Z. Kovari,
and G. Naray-Szabo, J. Chem. Soc. Perkin Trans., 2, 2231 (1996).
273 V. K. Yadav, J. Org. Chem., 66, 2501 (2001); V. K. Yadav and R. Balmurugan, J. Chem. Soc. Perkin
Trans., 2, 1 (2001).
274
C. K. Cheung, L. T. Tseng, M.-H. Lin, S. Srivastava and W. J. Le Noble, J. Am. Chem. Soc., 108, 1598
(1986); J. M. Hahn and W. J. Le Noble, J. Am. Chem. Soc., 114, 1916 (1992).
275 I. H. Song and W. J. Le Noble, J. Org. Chem., 59, 58 (1994).