Page 261 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 261
e. methyl 1S-cyano-2R-phenylcyclopropanecarboxylate 241
f. Z-2-methyl-2-buten-1-ol
PROBLEMS
g. E-(3-methyl-2-pentenylidene)triphenylphosphorane
2.4. Draw the structures of the product(s) described for each reaction. Specify all
aspects of the stereochemistry.
a. stereospecific anti addition of bromine to cis- and trans-cinnamic acid.
b. methanolysis of S-3-bromooctane with 6% racemization.
c. stereospecific syn thermal elimination of acetic acid from 1R,2S-
diphenylpropyl acetate
d. stereoselective epoxidation of bicyclo[2.2.1]hept-2-ene proceeding 94% from
the exo face.
2.5. The preferred conformation of 1-methyl-1-phenylcyclohexane has the phenyl
group in the axial orientation G =−0 32kcal/mol even though its confor-
mational free energy (2.9 kcal/mol) is greater than that of methyl (1.8 kcal/mol).
Explain.
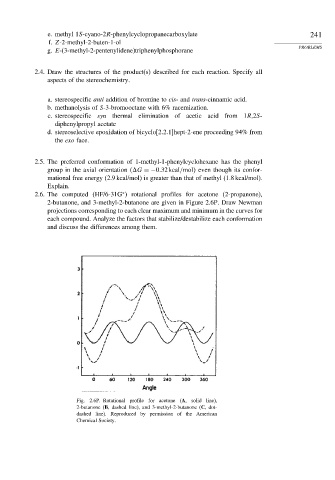
2.6. The computed (HF/6-31G ) rotational profiles for acetone (2-propanone),
∗
2-butanone, and 3-methyl-2-butanone are given in Figure 2.6P. Draw Newman
projections corresponding to each clear maximum and minimum in the curves for
each compound. Analyze the factors that stabilize/destabilize each conformation
and discuss the differences among them.
Fig. 2.6P. Rotational profile for acetone (A, solid line),
2-butanone (B, dashed line), and 3-methyl-2-butanone (C, dot-
dashed line). Reproduced by permission of the American
Chemical Society.