Page 252 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 252
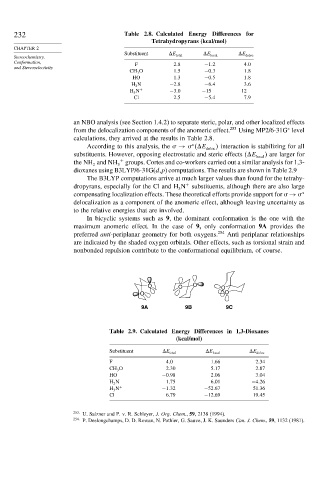
232 Table 2.8. Calculated Energy Differences for
Tetrahydropyrans (kcal/mol)
CHAPTER 2
Substituent E total E local E deloc
Stereochemistry,
Conformation, F 2 8 −1 2 4 0
and Stereoselectivity
CH 3 O 1 5 −0 3 1 8
HO 1 3 −0 5 1 8
H 2 N −2 8 −6 4 3 6
H 3 N + −3 0 −15 12
Cl 2 5 −5 4 7 9
an NBO analysis (see Section 1.4.2) to separate steric, polar, and other localized effects
∗
from the delocalization components of the anomeric effect. 253 Using MP2/6-31G level
calculations, they arrived at the results in Table 2.8.
According to this analysis, the → E deloc interaction is stabilizing for all
∗
substituents. However, opposing electrostatic and steric effects E local are larger for
the NH and NH 3 + groups. Cortes and co-workers carried out a similar analysis for 1,3-
2
dioxanes using B3LYP/6-31G(d,p) computations. The results are shown in Table 2.9
The B3LYP computations arrive at much larger values than found for the tetrahy-
dropyrans, especially for the Cl and H N substituents, although there are also large
+
3
compensating localization effects. These theoretical efforts provide support for → ∗
delocalization as a component of the anomeric effect, although leaving uncertainty as
to the relative energies that are involved.
In bicyclic systems such as 9, the dominant conformation is the one with the
maximum anomeric effect. In the case of 9, only conformation 9A provides the
preferred anti-periplanar geometry for both oxygens. 254 Anti periplanar relationships
are indicated by the shaded oxygen orbitals. Other effects, such as torsional strain and
nonbonded repulsion contribute to the conformational equilibrium, of course.
O O O O
O O
9A 9B 9C
Table 2.9. Calculated Energy Differences in 1,3-Dioxanes
(kcal/mol)
Substituent E total E local E deloc
F 4 0 1 66 2 34
CH 3 O 2 30 5 17 2 87
HO −0 98 2 06 3 04
H 2 N 1 75 6 01 −4 26
H 3 N + −1 32 −52 67 51 36
Cl 6 79 −12 69 19 45
253 U. Salzner and P. v. R. Schleyer, J. Org. Chem., 59, 2138 (1994).
254
P. Deslongchamps, D. D. Rowan, N. Pothier, G. Sauve, J. K. Saunders Can. J. Chem., 59, 1132 (1981).