Page 241 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 241
selectivity. Lipases from Candida rugosa (formerly Candida cylindracea), C. antartica, 221
and C. lipolytica are also used frequently. 222 Like the esterases, lipases can act as
hydrolysis catalysts toward esters or as acylation catalysts toward alcohols. Unlike TOPIC 2.2
PLE, the lipases have a specific type of natural substrate, namely triacyl glycerides. Enzymatic Resolution
and Desymmetrization
They are somewhat more selective in terms of substrate than PLE. Generally, neither
, -disubstituted carboxylates nor esters of tertiary alcohols are accepted as substrates.
As with PLE, either kinetic resolution or desymmetrization of prochiral reactants can
be achieved. The enantioselectivity of lipases depends upon discrimination between
the enantiomeric substrates at the active site. A large hydrophobic site acts as the
receptor for the largest nonpolar substituent.
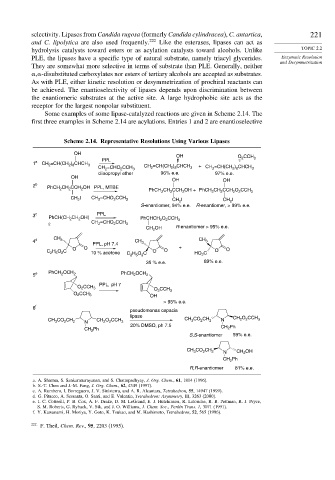
Some examples of some lipase-catalyzed reactions are given in Scheme 2.14. The
first three examples in Scheme 2.14 are acylations. Entries 1 and 2 are enantioselective
Scheme 2.14. Representative Resolutions Using Various Lipases
OH
OH O CCH
PPL 2 3
1 a CH 2 =CH(CH ) CHCH
2 8 3 CH =CH(CH ) CHCH + CH =CH(CH ) CHCH
CH =CHO CCH 3 2 2 8 3 2 2 8 3
2
2
diisopropyl ether 96% e.e. 97% e.e.
OH
OH OH
2 b CH CCH OH PPL, MTBE
PhCH 2 2 2 PhCH CH CCH OH + PhCH CH CCH O CCH 3
2
2
2
2
2
2
2
I CH =CHO CCH
CH 2 2 2 3 CH 2 I CH I
2
S-enantiomer, 94% e.e. R-enantiomer, > 99% e.e.
3 c PhCH(CH CH OH) PPL PhCHCH O CCH
2 2 2 2 3
CH =CHO CCH
2 2 2 3
CH OH R-enantiomer > 95% e.e.
2
CH CH
4 d 3 CH 3
PPL, pH 7.4 3
O O + O
C H O C 10 % acetone C H O C O O HO C O
2
2 5
2
2
2 5
35 % e.e. 89% e.e.
2
5 e PhCH OCH 2 PhCH OCH 2
2
PPL, pH 7
O 2 CCH 3 CCH
O 2 3
O 2 CCH 3
OH
> 95% e.e.
6 f
pseudomonas cepacia
lipase
2
CH CO CH 2 N CH 2 O CCH 3 CH CO CH 2 N CH O CCH 3
2
3
2
2
2
3
20% DMSO, ph 7.5
CH Ph CH 2 Ph
2
S,S-enantiomer 59% e.e.
CO CH
CH 3 2 2 N CH 2 OH
Ph
CH 2
R,R-enantiomer 81% e.e.
a. A. Sharma, S. Sankaranarayanan, and S. Chattopadhyay, J. Org. Chem., 61, 1814 (1996).
b. S.-T. Chen and J.-M. Fang, J. Org. Chem., 62, 4349 (1997).
c. A. Rumbero, I. Borreguero, J. V. Sinisterra, and A. R. Alcantara, Tetrahedron, 55, 14947 (1999).
d. G. Pitacco, A. Sessanta, O. Santi, and E. Valentin, Tetrahedron: Asymmetry, 11, 3263 (2000).
e. I. C. Cotterill, P. B. Cox, A. F. Drake, D. M. LeGrand, E. J. Hutchinson, R. Latouche, R. B. Pettman, R. J. Pryce,
S. M. Roberts, G. Ryback, V. Sik, and J. O. Williams, J. Chem. Soc., Perkin Trans. 1, 3071 (1991).
f. Y. Kawanami, H. Moriya, Y. Goto, K. Tsukao, and M. Hashimoto, Tetrahedron, 52, 565 (1996).
222
F. Theil, Chem. Rev., 95, 2203 (1995).