Page 227 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 227
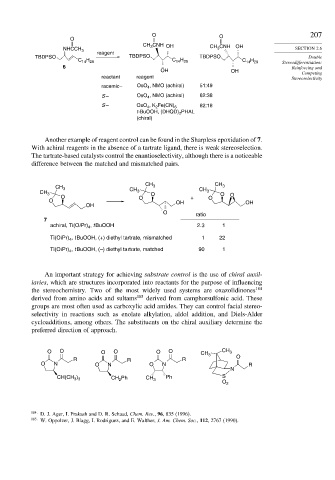
O O 207
O
CH 3 CNH OH CH 3 CNH OH
NHCCH 3 SECTION 2.6
reagent
TBDPSO TBDPSO TBDPSO Double
C 14 H 29 C 14 H 29 C 14 H 29
Stereodifferentiation:
6 Reinforcing and
OH OH Competing
reactant reagent Stereoselectivity
racemic– OsO 4 , NMO (achiral) 51:49
S – OsO 4 , NMO (achiral) 62:38
S – OsO 4 , K 2 Fe(CN) 6 82:18
t-BuOOH, (DHQD) 2 PHAL
(chiral)
Another example of reagent control can be found in the Sharpless epoxidation of 7.
With achiral reagents in the absence of a tartrate ligand, there is weak stereoselection.
The tartrate-based catalysts control the enantioselectivity, although there is a noticeable
difference between the matched and mismatched pairs.
CH CH
CH 3 3 3
CH 3 CH 3 O CH 3 O
O O + O O
O OH OH
OH
O
ratio
7
achiral, Ti(Oi Pr) , t BuOOH 2.3 1
4
Ti(Oi Pr) , t BuOOH, (+) diethyl tartrate, mismatched 1 22
4
Ti(Oi Pr) , t BuOOH, (–) diethyl tartrate, matched 90 1
4
An important strategy for achieving substrate control is the use of chiral auxil-
iaries, which are structures incorporated into reactants for the purpose of influencing
the stereochemistry. Two of the most widely used systems are oxazolidinones 184
derived from amino acids and sultams 185 derived from camphorsulfonic acid. These
groups are most often used as carboxylic acid amides. They can control facial stereo-
selectivity in reactions such as enolate alkylation, aldol addition, and Diels-Alder
cycloadditions, among others. The substituents on the chiral auxiliary determine the
preferred direction of approach.
O O O O O O CH 3 CH 3
R R R O
O N O N O N R
N
) Ph S
CH(CH 3 3 CH 2 Ph CH 3
O 2
184 D. J. Ager, I. Prakash and D. R. Schaad, Chem. Rev., 96, 835 (1996).
185
W. Oppolzer, J. Blagg, I. Rodriguez, and E. Walther, J. Am. Chem. Soc., 112, 2767 (1990).