Page 226 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 226
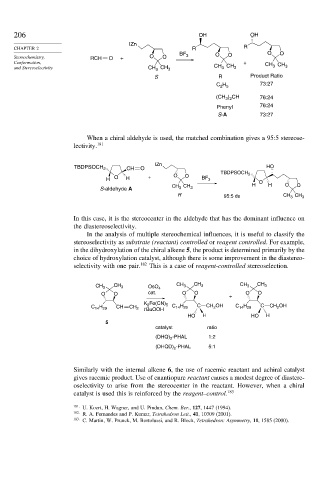
206 OH OH
IZn
CHAPTER 2 R R
BF O O O O
Stereochemistry, RCH O + O O 3
Conformation, + CH CH
and Stereoselectivity CH CH 3 CH 3 CH 3 3 3
3
S R Product Ratio
C H 73:27
2 5
(CH ) CH 76:24
3 2
Phenyl 76:24
S-A 73:27
When a chiral aldehyde is used, the matched combination gives a 95:5 stereose-
lectivity. 181
IZn
TBDPSOCH 2 CH O HO
TBDPSOCH 2
H O H + O O BF 3 O
CH H H O O
S-aldehyde A CH 3 3
R 95:5 ds CH 3 CH 3
In this case, it is the stereocenter in the aldehyde that has the dominant influence on
the diastereoselectivity.
In the analysis of multiple stereochemical influences, it is useful to classify the
stereoselectivity as substrate (reactant) controlled or reagent controlled. For example,
in the dihydroxylation of the chiral alkene 5, the product is determined primarily by the
choice of hydroxylation catalyst, although there is some improvement in the diastereo-
selectivity with one pair. 182 This is a case of reagent-controlled stereoselection.
CH 3 CH 3 OsO 4 CH 3 CH 3 CH 3 CH 3
O O cat. O O O O
+
K 2 Fe(CN) 5 C CH 2 OH C CH 2 OH
C 14 H 29 CH CH 2 C 14 H 29 C 14 H 29
t BuOOH
HO H HO H
5
catalyst ratio
(DHQ) 2 -PHAL 1:2
(DHQD) 2 -PHAL 5:1
Similarly with the internal alkene 6, the use of racemic reactant and achiral catalyst
gives racemic product. Use of enantiopure reactant causes a modest degree of diastere-
oselectivity to arise from the stereocenter in the reactant. However, when a chiral
catalyst is used this is reinforced by the reagent–control. 183
181
U. Koert, H. Wagner, and U. Pindun, Chem. Ber., 127, 1447 (1994).
182 R. A. Fernandes and P. Kumar, Tetrahedron Lett., 41, 10309 (2001).
183
C. Martin, W. Prunck, M. Bortolussi, and R. Bloch, Tetrahedron: Asymmetry, 11, 1585 (2000).