Page 229 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 229
209
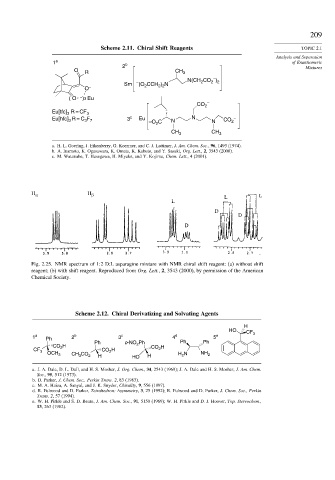
Scheme 2.11. Chiral Shift Reagents TOPIC 2.1
Analysis and Separation
1 a b of Enantiomeric
2 Mixtures
O R CH 3
–
N(CH CO )
Sm – (O CCH ) N 2 2 2
O – 2 2 2
( O )3 Eu
CO 2 –
Eu[tfc] R = CF 3
3
Eu[hfc] R = C F 3 c Eu –O C N N N CO 2 –
3 7
3
2
CH 3 CH 3
a. H. L. Goering, J. Eikenberry, G. Koerrner, and C. J. Lattimer, J. Am. Chem. Soc., 96, 1493 (1974).
b. A. Inamoto, K. Ogasawara, K. Omata, K. Kabuto, and Y. Sasaki, Org. Lett., 2, 3543 (2000).
c. M. Watanabe, T. Hasegawa, H. Miyake, and Y. Kojima, Chem. Lett., 4 (2001).
H α H β
Fig. 2.25. NMR spectrum of 1:2 D:L asparagine mixture with NMR chiral shift reagent: (a) without shift
reagent; (b) with shift reagent. Reproduced from Org. Lett., 2, 3543 (2000), by permission of the American
Chemical Society.
Scheme 2.12. Chiral Derivatizing and Solvating Agents
H
HO
CF 3
1 a Ph 2 b 3 c 4 d 5 e
Ph o-NO Ph Ph Ph
H 2
CO 2 CO H
CF CO H 2
3
OCH 3 CH CO 2 H 2 HO H H N NH 2
2
3
a. J. A. Dale, D. L. Dull, and H. S. Mosher, J. Org. Chem., 34, 2543 (1969); J. A. Dale and H. S. Mosher, J. Am. Chem.
Soc., 95, 512 (1973).
b. D. Parker, J. Chem. Soc., Perkin Trans. 2, 83 (1983).
c. M. A. Haiza, A. Sanyal, and J. K. Snyder, Chirality, 9, 556 (1997).
d. R. Fulwood and D. Parker, Tetrahedron: Asymmetry, 3, 25 (1992); R. Fulwood and D. Parker, J. Chem. Soc., Perkin
Trans. 2, 57 (1994).
e. W. H. Pirkle and S. D. Beare, J. Am. Chem. Soc., 91, 5150 (1969); W. H. Pirkle and D. J. Hoover, Top. Stereochem.,
13, 263 (1982).