Page 549 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 549
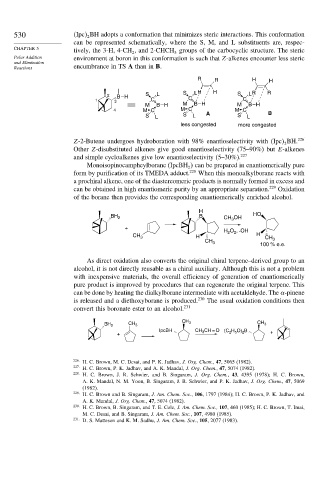
530 Ipc BH adopts a conformation that minimizes steric interactions. This conformation
2
can be represented schematically, where the S, M, and L substituents are, respec-
CHAPTER 5
tively, the 3-H, 4-CH , and 2-CHCH groups of the carbocyclic structure. The steric
2
3
Polar Addition environment at boron in this conformation is such that Z-alkenes encounter less steric
and Elimination
Reactions encumbrance in TS A than in B.
R R H H
2 BH S L S L H H S L R R
1 3 C C C
M BH M BH M BH
4 M C M C M C
S L S L A S L B
less congested more congested
Z-2-Butene undergoes hydroboration with 98% enantioselectivity with Ipc BH. 226
2
Other Z-disubstituted alkenes give good enantioselectivity (75–90%) but E-alkenes
and simple cycloalkenes give low enantioselectivity (5–30%). 227
Monoisopinocampheylborane (IpcBH can be prepared in enantiomerically pure
2
form by purification of its TMEDA adduct. 228 When this monoalkylborane reacts with
a prochiral alkene, one of the diastereomeric products is normally formed in excess and
can be obtained in high enantiomeric purity by an appropriate separation. 229 Oxidation
of the borane then provides the corresponding enantiomerically enriched alcohol.
H
BH 2 B CH OH HO
3
+
H O , -OH
2
2
CH 3 H H CH
CH 3 3
100 % e.e.
As direct oxidation also converts the original chiral terpene–derived group to an
alcohol, it is not directly reusable as a chiral auxiliary. Although this is not a problem
with inexpensive materials, the overall efficiency of generation of enantiomerically
pure product is improved by procedures that can regenerate the original terpene. This
can be done by heating the dialkylborane intermediate with acetaldehyde. The -pinene
is released and a diethoxyborane is produced. 230 The usual oxidation conditions then
convert this boronate ester to an alcohol. 231
CH 3
BH 2 CH 3 CH 3
IpcBH CH 3 CH = O (C 2 H 5 O) 2 B
+ +
226
H. C. Brown, M. C. Desai, and P. K. Jadhav, J. Org. Chem., 47, 5065 (1982).
227
H. C. Brown, P. K. Jadhav, and A. K. Mandal, J. Org. Chem., 47, 5074 (1982).
228 H. C. Brown, J. R. Schwier, and B. Singaram, J. Org. Chem., 43, 4395 (1978); H. C. Brown,
A. K. Mandal, N. M. Yoon, B. Singaram, J. R. Schwier, and P. K. Jadhav, J. Org. Chem., 47, 5069
(1982).
229
H. C. Brown and B. Singaram, J. Am. Chem. Soc., 106, 1797 (1984); H. C. Brown, P. K. Jadhav, and
A. K. Mandal, J. Org. Chem., 47, 5074 (1982).
230 H. C. Brown, B. Singaram, and T. E. Cole, J. Am. Chem. Soc., 107, 460 (1985); H. C. Brown, T. Imai,
M. C. Desai, and B. Singaram, J. Am. Chem. Soc., 107, 4980 (1985).
231
D. S. Matteson and K. M. Sadhu, J. Am. Chem. Soc., 105, 2077 (1983).