Page 551 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 551
532
10.5 10.0 9.5 9.0 8.5 Ph 8.0
CHAPTER 5 9.24 9.11 8.27
Polar Addition 10.51 9.73 8.69 8.46
and Elimination 11.0 9.10
Reactions
10.0
9.0
8.0
7.0
6.0
5.0
4.0
3.0
2.0
1.0
0.0
–1.0
–2.0
protonation
bromination (Br 2 )
–3.0
sulfenylation (ArSCl)
selenenylation (ArSeCl)
–4.0
epoxidation (CH 3 CO 3 H)
mercuration (Hg(OAc) 2 )
–5.0 hydroboration (9BBN)
10.5 10.0 9.5 9.0 8.5 8.0
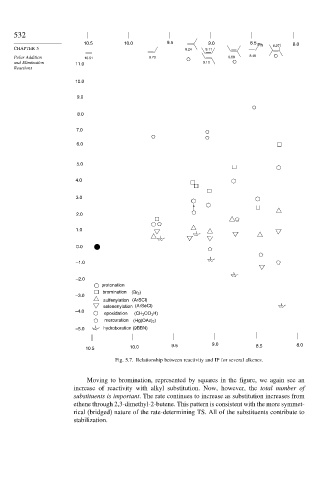
Fig. 5.7. Relationship between reactivity and IP for several alkenes.
Moving to bromination, represented by squares in the figure, we again see an
increase of reactivity with alkyl substitution. Now, however, the total number of
substituents is important. The rate continues to increase as substitution increases from
ethene through 2,3-dimethyl-2-butene. This pattern is consistent with the more symmet-
rical (bridged) nature of the rate-determining TS. All of the substituents contribute to
stabilization.