Page 550 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 550
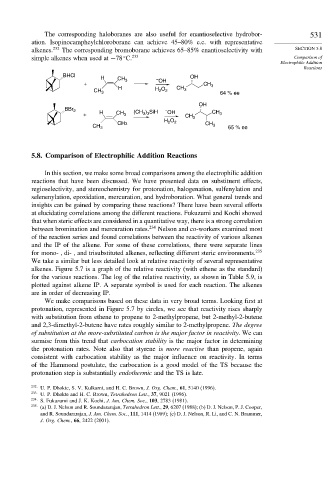
The corresponding haloboranes are also useful for enantioselective hydrobor- 531
ation. Isopinocampheylchloroborane can achieve 45–80% e.e. with representative
alkenes. 232 The corresponding bromoborane achieves 65–85% enantioselectivity with SECTION 5.8
simple alkenes when used at −78 C. 233 Comparison of
Electrophilic Addition
Reactions
BHCl OH
H CH 3 –
+ OH CH 3
H O CH
CH 3 H 2 2 3 64 % ee
OH
BBr 2 (CH ) SiH –
+ H CH 3 3 3 OH CH CH 3
H O 3
CH3 2 2 CH
CH 3 3 65 % ee
5.8. Comparison of Electrophilic Addition Reactions
In this section, we make some broad comparisons among the electrophilic addition
reactions that have been discussed. We have presented data on substituent effects,
regioselectivity, and stereochemistry for protonation, halogenation, sulfenylation and
selenenylation, epoxidation, mercuration, and hydroboration. What general trends and
insights can be gained by comparing these reactions? There have been several efforts
at elucidating correlations among the different reactions. Fukuzumi and Kochi showed
that when steric effects are considered in a quantitative way, there is a strong correlation
between bromination and mercuration rates. 234 Nelson and co-workers examined most
of the reaction series and found correlations between the reactivity of various alkenes
and the IP of the alkene. For some of these correlations, there were separate lines
for mono- , di- , and trisubstituted alkenes, reflecting different steric environments. 235
We take a similar but less detailed look at relative reactivity of several representative
alkenes. Figure 5.7 is a graph of the relative reactivity (with ethene as the standard)
for the various reactions. The log of the relative reactivity, as shown in Table 5.9, is
plotted against alkene IP. A separate symbol is used for each reaction. The alkenes
are in order of decreasing IP.
We make comparisons based on these data in very broad terms. Looking first at
protonation, represented in Figure 5.7 by circles, we see that reactivity rises sharply
with substitution from ethene to propene to 2-methylpropene, but 2-methyl-2-butene
and 2,3-dimethyl-2-butene have rates roughly similar to 2-methylpropene. The degree
of substitution at the more-substituted carbon is the major factor in reactivity. We can
surmise from this trend that carbocation stability is the major factor in determining
the protonation rates. Note also that styrene is more reactive than propene, again
consistent with carbocation stability as the major influence on reactivity. In terms
of the Hammond postulate, the carbocation is a good model of the TS because the
protonation step is substantially endothermic and the TS is late.
232
U. P. Dhokte, S. V. Kulkarni, and H. C. Brown, J. Org. Chem., 61, 5140 (1996).
233
U. P. Dhokte and H. C. Brown, Tetrahedron Lett., 37, 9021 (1996).
234 S. Fukuzumi and J. K. Kochi, J. Am. Chem. Soc., 103, 2783 (1981).
235
(a) D. J. Nelson and R. Soundararajan, Tetrahedron Lett., 29, 6207 (1988); (b) D. J. Nelson, P. J. Cooper,
and R. Soundararajan, J. Am. Chem. Soc., 111, 1414 (1989); (c) D. J. Nelson, R. Li, and C. N. Brammer,
J. Org. Chem., 66, 2422 (2001).