Page 552 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 552
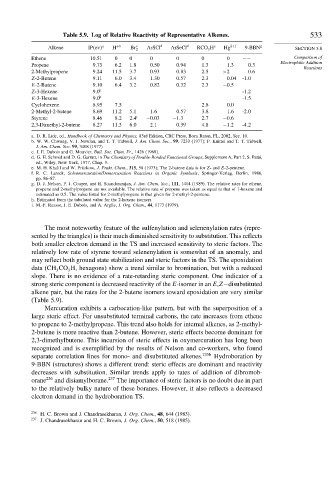
Table 5.9. Log of Relative Reactivity of Representative Alkenes. 533
Alkene IP ev a H +b Br c 2 ArSCl d ArSeCl d RCO 3 H e Hg 2+f 9-BBN g SECTION 5.8
Ethene 10 51 0 0 0 0 0 0 −− Comparison of
Electrophilic Addition
Propene 9 73 6 2 1 8 0 50 0 94 1 3 1 3 0 3
Reactions
2-Methylpropene 9 24 11 5 3 7 0 93 0 83 2 5 >2 0 6
Z-2-Butene 9 11 6 0 3 4 1 30 0 57 2 3 0 04 -1 0
E-2-Butene 9 10 6 4 3 2 0 82 0 32 2 3 −0 5
Z-3-Hexene 9 0 h -1 2
E-3-Hexene 9 0 h -1 5
Cyclohexene 8 95 7 5 2 8 0 0
2-Methyl-2-butene 8 69 11 2 5 1 1 6 0 57 3 8 1 6 -2 0
Styrene 8 46 8 2 2 4 i −0 03 −1 3 2 7 −0 6
2,3-Dimethyl-2-butene 8 27 11 3 6 0 2 1 0 39 4 8 −1 2 -4 2
a. D. R. Lide, ed., Handbook of Chemistry and Physics, 83rd Edition, CRC Press, Boca Raton, FL, 2002, Sec. 10.
b. W. W. Chwang, V. J. Nowlan, and T. T. Tidwell, J. Am. Chem. Soc., 99, 7233 (1977); P. Knittel and T. T. Tidwell,
J. Am. Chem. Soc. 99, 3408 (1977)
c. J. E. Dubois and G. Mouvier, Bull. Soc. Chim. Fr., 1426 (1968).
d. G. H. Schmid and D. G. Garratt, in The Chemistry of Double-Bonded Functional Groups, Supplement A, Part 2, S. Patai,
ed., Wiley, New York, 1977, Chap. 9.
e. M. H. Khalil and W. Pritzkow, J. Prakt. Chem., 315, 58 (1973); The 2-butene data is for Z- and E-2-pentene.
f. R. C. Larock, Solvomercuration/Demercuration Reactions in Organic Synthesis, Springer-Verlag, Berlin, 1986,
pp. 86–87.
g. D. J. Nelson, P. J. Cooper, and R. Soundararajan, J. Am. Chem. Soc., 111, 1414 (1989). The relative rates for ethene,
propene and 2-methylpropene are not available. The relative rate of propene was taken as equal to that of 1-hexene and
estimated as 0.5. The value listed for 2-methylpropene is that given for 2-methyl-2-pentene.
h. Estimated from the tabulated value for the 2-hexene isomers.
i. M.-F. Ruasse, J. E. Dubois, and A. Argile, J. Org. Chem., 44, 1173 (1979).
The most noteworthy feature of the sulfenylation and selenenylation rates (repre-
sented by the triangles) is their much diminished sensitivity to substitution. This reflects
both smaller electron demand in the TS and increased sensitivity to steric factors. The
relatively low rate of styrene toward selenenylation is somewhat of an anomaly, and
may reflect both ground state stabilization and steric factors in the TS. The epoxidation
data (CH CO H, hexagons) show a trend similar to bromination, but with a reduced
3
3
slope. There is no evidence of a rate-retarding steric component. One indicator of a
strong steric component is decreased reactivity of the E-isomer in an E,Z−disubstituted
alkene pair, but the rates for the 2-butene isomers toward epoxidation are very similar
(Table 5.9).
Mercuration exhibits a carbocation-like pattern, but with the superposition of a
large steric effect. For unsubstituted terminal carbons, the rate increases from ethene
to propene to 2-methylpropene. This trend also holds for internal alkenes, as 2-methyl-
2-butene is more reactive than 2-butene. However, steric effects become dominant for
2,3-dimethylbutene. This incursion of steric effects in oxymercuration has long been
recognized and is exemplified by the results of Nelson and co-workers, who found
separate correlation lines for mono- and disubstituted alkenes. 235b Hydroboration by
9-BBN (structures) shows a different trend: steric effects are dominant and reactivity
decreases with substitution. Similar trends apply to rates of addition of dibromob-
orane 236 and disiamylborane. 237 The importance of steric factors is no doubt due in part
to the relatively bulky nature of these boranes. However, it also reflects a decreased
electron demand in the hydroboration TS.
236 H. C. Brown and J. Chandrasekharan, J. Org. Chem., 48, 644 (1983).
237
J. Chandrasekharan and H. C. Brown, J. Org. Chem., 50, 518 (1985).