Page 553 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 553
534 An overall perspective of the data in Table 5.9 and Figure 5.7 can be gained
by simply comparing the relative rates of ethene and 2,3-dimethyl-2-butene. These
CHAPTER 5 go from very large for protonation (10 11 4 ) to small (10 −4 2 ) for hydroboration by
Polar Addition 9-BBN as the dominance of carbocation stability effects for protonation is replaced by
and Elimination
Reactions dominant steric effects for hydroboration by 9-BBN.
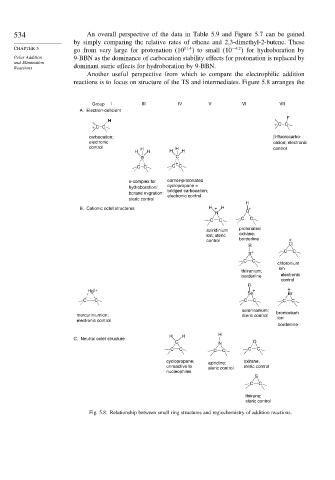
Another useful perspective from which to compare the electrophilic addition
reactions is to focus on structure of the TS and intermediates. Figure 5.8 arranges the
Group I III IV V VI VII
A. Electron-deficient
F
H
+ CC
+ CC
carbocation; β-fluorocarbo-
electronic cation; electronic
control H H control
H H H H
B C
+
CC CC
π-complex for corner-protonated
hydroboration/ cyclopropane =
bridged carbocation;
borane migration:
electronic control
steric control
H
B. Cationic octet structures H + H +
N O
C C
C C
protonated
aziridinium
ion; steric oxirane;
control borderline +
R Cl
+ C C
S
C C
chloronium
ion
thiiranium;
borderline electronic
control
R
Hg 2+ + +
Se Br
C C C C C C
seleniranium; bromonium
mercuriniumion; steric control ion
electronic control
borderline
H
H H
C. Neutral octet structure
C N O
C C C C C C
cyclopropane; aziridine; oxirane;
unreactive to steric control steric control
nucleophiles
S
C C
thiirane;
steric control
Fig. 5.8. Relationship between small ring structures and regiochemistry of addition reactions.