Page 554 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 554
cationic and neutral species in relation to the periodic table. Some of these structures 535
are stable products, others are reaction intermediates, and still other are transition
structures. If the bridged and open versions of a species are comparable in energy, SECTION 5.8
both are shown. Comparison of
Electrophilic Addition
Taking the view that protonation of alkenes gives open carbocations, at least Reactions
in solution, gives us a starting point for comparing the various addition reaction
intermediates. From the discussion of carbocations in Chapter 4, we know the reactions
that are characteristic of carbocations, namely nucleophilic capture, elimination of
a proton, and rearrangement. These reactions are controlled by electronic factors,
as illustrated by Markovnikov regioselectivity for nucleophile capture and Saytzeff
regiochemistry for elimination. (see Section 4.4.3) The TSs for hydroboration and
protonated cyclopropanes are related to open carbocations in being electron deficient.
The hydroboration TS collapses by a hydride shift from boron to carbon that is
driven by both electronegativity and steric factors. Corner-protonated cyclopropanes
are converted to the carbocation best able to support positive charge. It is also clear
from the mercuration reaction that a ligand transfer process is available when anti-
nucleophilic addition is sterically hindered. The syn addition is similar to the hydride
migration that occurs during hydroboration. The regiochemistry of the migration is
dictated by electronic factors.
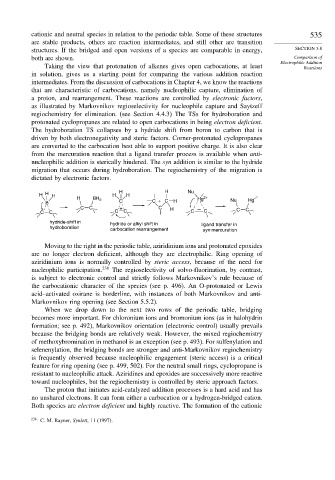
H H H H H H H Nu
H BH 2 Hg 2+ Nu Hg
B C C + C H
C C + C H C C
C C C C C C
hydride-shift in hydride or alkyl shift in ligand transfer in
hydroboration carbocation rearrangement syn mercuration
Moving to the right in the periodic table, aziridinium ions and protonated epoxides
are no longer electron deficient, although they are electrophilic. Ring opening of
aziridinium ions is normally controlled by steric access, because of the need for
nucleophilic participation. 238 The regioselectivity of solvo-fluorination, by contrast,
is subject to electronic control and strictly follows Markovnikov’s rule because of
the carbocationic character of the species (see p. 496). An O-protonated or Lewis
acid–activated oxirane is borderline, with instances of both Markovnikov and anti-
Markovnikov ring opening (see Section 5.5.2).
When we drop down to the next two rows of the periodic table, bridging
becomes more important. For chloronium ions and bromonium ions (as in halohydrin
formation; see p. 492), Markovnikov orientation (electronic control) usually prevails
because the bridging bonds are relatively weak. However, the mixed regiochemistry
of methoxybromination in methanol is an exception (see p. 493). For sulfenylation and
selenenylation, the bridging bonds are stronger and anti-Markovnikov regiochemistry
is frequently observed because nucleophilic engagement (steric access) is a critical
feature for ring opening (see p. 499, 502). For the neutral small rings, cyclopropane is
resistant to nucleophilic attack. Aziridines and epoxides are successively more reactive
toward nucleophiles, but the regiochemistry is controlled by steric approach factors.
The proton that initiates acid-catalyzed addition processes is a hard acid and has
no unshared electrons. It can form either a carbocation or a hydrogen-bridged cation.
Both species are electron deficient and highly reactive. The formation of the cationic
238
C. M. Rayner, Synlett, 11 (1997).