Page 728 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 728
710 0.0
CHAPTER 7
Addition, Condensation
and Substitution –2.5
log k obs (s –1 ) –5.0
Reactions of Carbonyl
Compounds
–7.5
0.0 2.5 5.0 7.5 10.0
pH
Fig. 7.P28. pH-Rate plots for thioesters 28-A ( ),28-B (•),28-C( ) and
28-D( ). Reproduced from J. Org. Chem., 62, 4816 (1997), by permission
of the American Chemical Society.
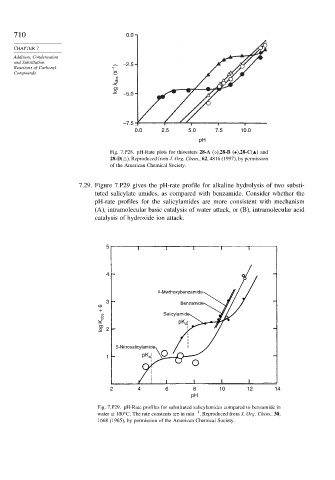
7.29. Figure 7.P29 gives the pH-rate profile for alkaline hydrolysis of two substi-
tuted salicylate amides, as compared with benzamide. Consider whether the
pH-rate profiles for the salicylamides are more consistent with mechanism
(A), intramolecular basic catalysis of water attack, or (B), intramolecular acid
catalysis of hydroxide ion attack.
5
4
º -Mwthoxybenzamide
3 Benzamide
log K obs + 6 2 Salicylamide
pK
a
5-Nitrosalicylamide
1 pK a
2 4 6 8 10 12 14
pH
Fig. 7.P29. pH-Rate profiles for substituted salicylamides compared to benzamide in
−1
water at 100 C. The rate constants are in min . Reproduced from J. Org. Chem.. 30,
1668 (1965), by permission of the American Chemical Society.