Page 724 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 724
706
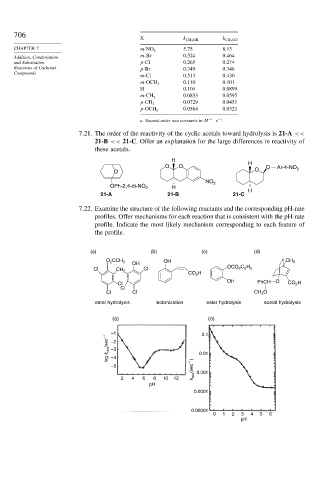
X k CH 3 OH k CH 3 OD
CHAPTER 7 m-NO 2 5.75 8.13
Addition, Condensation m-Br 0.524 0.464
and Substitution p-Cl 0.265 0.274
Reactions of Carbonyl p-Br 0.349 0.346
Compounds
m-Cl 0.513 0.430
0.110 0.101
m-OCH 3
H 0.104 0.0899
0.0833 0.0595
m-CH 3
0.0729 0.0451
p-CH 3
0.0564 0.0321
p-OCH 3
−1
a. Second-order rate constants in M −1 s .
7.21. The order of the reactivity of the cyclic acetals toward hydrolysis is 21-A <<
21-B << 21-C. Offer an explanation for the large differences in reactivity of
these acetals.
H
O O H O Ar-4-NO 2
O O
NO 2
OPh-2,4-di-NO 2 H
H
21-A 21-B 21-C
7.22. Examine the structure of the following reactants and the corresponding pH-rate
profiles. Offer mechanisms for each reaction that is consistent with the pH-rate
profile. Indicate the most likely mechanism corresponding to each feature of
the profile.
(a) (b) (c) (d)
O 2 CCH 3 OH CH 3
OH
Cl CH 2 Cl OCO 2 C 2 H 5
CO 2 H
OH PhCH O
Cl CO 2 H
Cl
Cl Cl CH 3 O
ester hydrolysis lactonization ester hydrolysis acetal hydrolysis
(a) (b)
–1 0.1
log k obs /sec –1 –2 0.01
–3
–4
–5 k obs (sec –1 ) 0.001
2 4 6 8 10 12
pH
0.0001
0.00001
0 1 2 3 4 5 6
pH