Page 727 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 727
709
PROBLEMS
–1
log k hydr min –1 –2
–3
0 2 4 6
pH
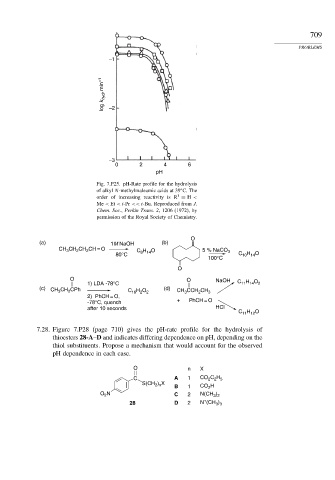
Fig. 7.P25. pH-Rate profile for the hydrolysis
of alkyl N-methylmaleamic acids at 39 C. The
1
order of increasing reactivity is R = H <
Me < Et <i-Pr << t-Bu. Reproduced from J.
Chem. Soc., Perkin Trans. 2, 1206 (1972), by
permission of the Royal Society of Chemistry.
O
(a) 1M NaOH (b)
CH CH CH CH = O C H O 5 % NaCO 3
2
2
3
80°C 8 14 C H O
10 14
100°C
O
O O NaOH
1) LDA -78°C C H O 2
11 14
(c) CH CH CPh C H O (d) CH CCH CH
3 2 16 2 2 3 2 3
2) PhCH = O,
-78°C, quench + PhCH = O
after 10 seconds HCl
C H O
11 12
7.28. Figure 7.P28 (page 710) gives the pH-rate profile for the hydrolysis of
thioesters 28-A–D and indicates differing dependence on pH, depending on the
thiol substituents. Propose a mechanism that would account for the observed
pH dependence in each case.
O n X
C H
C A 1 CO 2 2 5
S(CH ) X B 1 CO H
2 n
2
O N C 2 N(CH )
2
3 2
+
28 D 2 N (CH )
3 3