Page 723 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 723
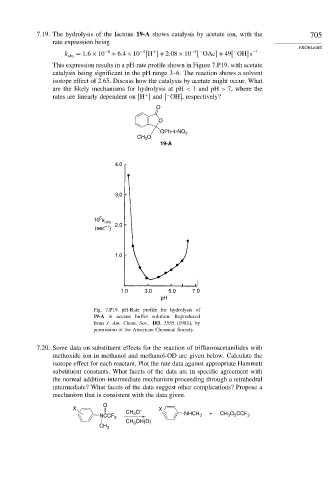
7.19. The hydrolysis of the lactone 19-A shows catalysis by acetate ion, with the 705
rate expression being
PROBLEMS
−4
−
−5 −
+
k = 1 6×10 −6 +6 4×10 H +2 08×10 OAc +49 OH s −1
obs
This expression results in a pH-rate profile shown in Figure 7.P19, with acetate
catalysis being significant in the pH range 3–6. The reaction shows a solvent
isotope effect of 2.65. Discuss how the catalysis by acetate might occur. What
are the likely mechanisms for hydrolysis at pH < 1 and pH > 7, where the
−
rates are linearly dependent on [H ] and [ OH], respectively?
+
O
O
OPh-4-NO 2
O
CH 3
19-A
4.0
3.0
5
10 k
obs 2.0
–1
(sec )
1.0
1.0 3.0 5.0 7.0
pH
Fig. 7.P19. pH-Rate profile for hydrolysis of
19-A in acetate buffer solution. Reproduced
from J. Am. Chem. Soc., 103, 3555 (1981), by
permission of the American Chemical Society.
7.20. Some data on substituent effects for the reaction of trifluoroacetanilides with
methoxide ion in methanol and methanol-OD are given below. Calculate the
isotope effect for each reactant. Plot the rate data against appropriate Hammett
substituent constants. What facets of the data are in specific agreement with
the normal addition-intermediate mechanism proceeding through a tetrahedral
intermediate? What facets of the data suggest other complications? Propose a
mechanism that is consistent with the data given.
O
X – X
CH 3 O +
NHCH 3 CH 3 O 2 CCF 3
NCCF 3
CH OH(D)
3
CH 3