Page 720 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 720
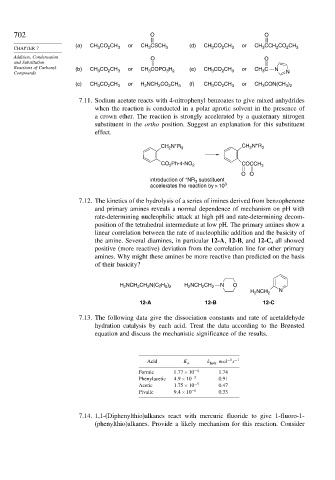
702 O O
(a) or (d) or
3
CHAPTER 7 CH 3 CO 2 CH 3 CH CSCH 3 CH 3 CO 2 CH 3 CH 3 CCH 2 CO 2 CH 3
Addition, Condensation O O
and Substitution
Reactions of Carbonyl (b) CH CO CH or CH COPO H (e) CH CO CH or CH C N
Compounds 3 2 3 3 3 2 3 2 3 3 N
(c) CH CO CH 3 or H NCH CO CH 3 (f) CH CO CH 3 or CH CON(CH )
3
2
2
2
2
3
3
3 2
2
7.11. Sodium acetate reacts with 4-nitrophenyl benzoates to give mixed anhydrides
when the reaction is conducted in a polar aprotic solvent in the presence of
a crown ether. The reaction is strongly accelerated by a quaternary nitrogen
substituent in the ortho position. Suggest an explanation for this substituent
effect.
+
+
CH N R 3 CH N R 3
2
2
CO Ph-4-NO 2 COCCH 3
2
OO
+
introduction of NR substituent
3
3
accelerates the reaction by > 10 .
7.12. The kinetics of the hydrolysis of a series of imines derived from benzophenone
and primary amines reveals a normal dependence of mechanism on pH with
rate-determining nucleophilic attack at high pH and rate-determining decom-
position of the tetrahedral intermediate at low pH. The primary amines show a
linear correlation between the rate of nucleophilic addition and the basicity of
the amine. Several diamines, in particular 12-A, 12-B, and 12-C, all showed
positive (more reactive) deviation from the correlation line for other primary
amines. Why might these amines be more reactive than predicted on the basis
of their basicity?
H NCH CH N(C 2H ) H NCH CH 2 N O
2
2
2
2
2
5 2
H 2 NCH 2 N
12-A 12-B 12-C
7.13. The following data give the dissociation constants and rate of acetaldehyde
hydration catalysis by each acid. Treat the data according to the Brønsted
equation and discuss the mechanistic significance of the results.
−1 −1
Acid K a k hydr mol s
Formic 1 77×10 −4 1.74
Phenylacetic 4 9×10 −5 0.91
Acetic 1 75×10 −5 0.47
Pivalic 9 4×10 −6 0.33
7.14. 1,1-(Diphenylthio)alkanes react with mercuric fluoride to give 1-fluoro-1-
(phenylthio)alkanes. Provide a likely mechanism for this reaction. Consider