Page 719 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 719
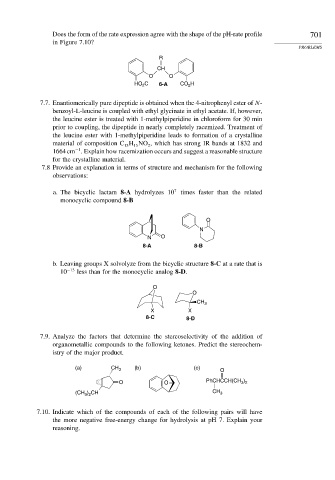
Does the form of the rate expression agree with the shape of the pH-rate profile 701
in Figure 7.10?
PROBLEMS
R
CH
O O
HO C 6-A CO 2 H
2
7.7. Enantiomerically pure dipeptide is obtained when the 4-nitrophenyl ester of N-
benzoyl-L-leucine is coupled with ethyl glycinate in ethyl acetate. If, however,
the leucine ester is treated with 1-methylpiperidine in chloroform for 30 min
prior to coupling, the dipeptide in nearly completely racemized. Treatment of
the leucine ester with 1-methylpiperidine leads to formation of a crystalline
material of composition C H NO , which has strong IR bands at 1832 and
13
15
2
−1
1664cm . Explain how racemization occurs and suggest a reasonable structure
for the crystalline material.
7.8 Provide an explanation in terms of structure and mechanism for the following
observations:
7
a. The bicyclic lactam 8-A hydrolyzes 10 times faster than the related
monocyclic compound 8-B
O
N
N O
8-A 8-B
b. Leaving groups X solvolyze from the bicyclic structure 8-C at a rate that is
10 −13 less than for the monocyclic analog 8-D.
O
O
CH 3
X X
8-C 8-D
7.9. Analyze the factors that determine the stereoselectivity of the addition of
organometallic compounds to the following ketones. Predict the stereochem-
istry of the major product.
(a) CH 3 (b) (c) O
)
O O PhCHCCH(CH 3 2
(CH 3 ) 2 CH CH 3
7.10. Indicate which of the compounds of each of the following pairs will have
the more negative free-energy change for hydrolysis at pH 7. Explain your
reasoning.