Page 717 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 717
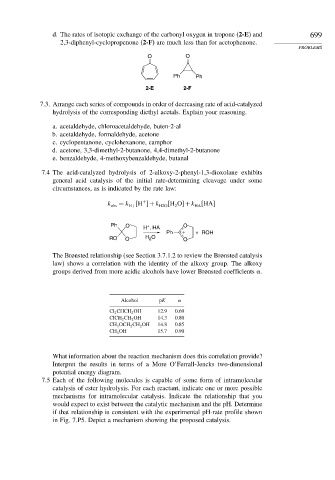
d. The rates of isotopic exchange of the carbonyl oxygen in tropone (2-E) and 699
2,3-diphenyl-cyclopropenone (2-F) are much less than for acetophenone.
PROBLEMS
O O
Ph Ph
2-E 2-F
7.3. Arrange each series of compounds in order of decreasing rate of acid-catalyzed
hydrolysis of the corresponding diethyl acetals. Explain your reasoning.
a. acetaldehyde, chloroacetaldehyde, buten-2-al
b. acetaldehyde, formaldehyde, acetone
c. cyclopentanone, cyclohexanone, camphor
d. acetone, 3,3-dimethyl-2-butanone, 4,4-dimethyl-2-butanone
e. benzaldehyde, 4-methoxybenzaldehyde, butanal
7.4 The acid-catalyzed hydrolysis of 2-alkoxy-2-phenyl-1,3-dioxolane exhibits
general acid catalysis of the initial rate-determining cleavage under some
circumstances, as is indicated by the rate law:
+
k = k H +k H O +k HA
obs H+ H2O 2 HA
Ph O H , HA O
+
Ph + + ROH
RO O H O O
2
The Brønsted relationship (see Section 3.7.1.2 to review the Brønsted catalysis
law) shows a correlation with the identity of the alkoxy group. The alkoxy
groups derived from more acidic alcohols have lower Brønsted coefficients
.
Alcohol pK
Cl 2 CHCH 2 OH 12.9 0.69
ClCH 2 CH 2 OH 14.3 0.80
CH 3 OCH 2 CH 2 OH 14.8 0.85
CH 3 OH 15.7 0.90
What information about the reaction mechanism does this correlation provide?
Interpret the results in terms of a More O’Ferrall-Jencks two-dimensional
potential energy diagram.
7.5 Each of the following molecules is capable of some form of intramolecular
catalysis of ester hydrolysis. For each reactant, indicate one or more possible
mechanisms for intramolecular catalysis. Indicate the relationship that you
would expect to exist between the catalytic mechanism and the pH. Determine
if that relationship is consistent with the experimental pH-rate profile shown
in Fig. 7.P5. Depict a mechanism showing the proposed catalysis.