Page 713 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 713
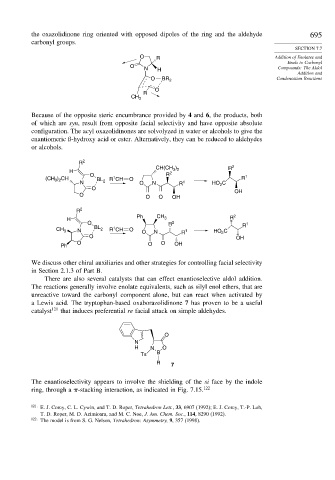
the oxazolidinone ring oriented with opposed dipoles of the ring and the aldehyde 695
carbonyl groups.
SECTION 7.7
O R Addition of Enolates and
Enols to Carbonyl
O
N H Compounds: The Aldol
Addition and
O BR 2 Condensation Reactions
O
R
CH 3
Because of the opposite steric encumbrance provided by 4 and 6, the products, both
of which are syn, result from opposite facial selectivity and have opposite absolute
configuration. The acyl oxazolidinones are solvolyzed in water or alcohols to give the
enantiomeric ß-hydroxy acid or ester. Alternatively, they can be reduced to aldehydes
or alcohols.
R 2
CH(CH ) R 2
H R 2 3 2
) CH O 1 R 1
(CH 3 2 BL 2 R CH O
N O N R 1 HO C
2
O
O O O OH OH
R 2
Ph CH 3 R 2
H
O R 2 R 1
1
CH 3 N BL 2 R CH O O N R 1 HO C
O 2 OH
O O
Ph O OH
We discuss other chiral auxiliaries and other strategies for controlling facial selectivity
in Section 2.1.3 of Part B.
There are also several catalysts that can effect enantioselective aldol addition.
The reactions generally involve enolate equivalents, such as silyl enol ethers, that are
unreactive toward the carbonyl component alone, but can react when activated by
a Lewis acid. The tryptophan-based oxaborazolidinone 7 has proven to be a useful
catalyst 121 that induces preferential re facial attack on simple aldehydes.
O
N
H N O
Ts B
R 7
The enantioselectivity appears to involve the shielding of the si face by the indole
ring, through a -stacking interaction, as indicated in Fig. 7.15. 122
121 E. J. Corey, C. L. Cywin, and T. D. Roper, Tetrahedron Lett., 33, 6907 (1992); E. J. Corey, T.-P. Loh,
T. D. Roper, M. D. Azimioara, and M. C. Noe, J. Am. Chem. Soc., 114, 8290 (1992).
122
The model is from S. G. Nelson, Tetrahedron: Asymmetry, 9, 357 (1998).