Page 709 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 709
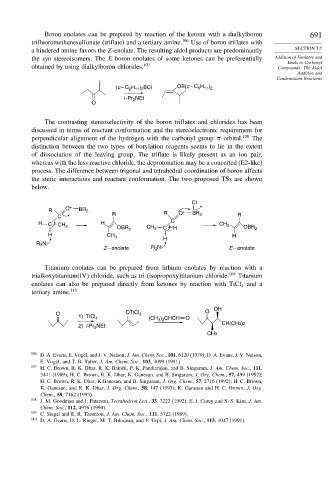
Boron enolates can be prepared by reaction of the ketone with a dialkylboron 691
trifluoromethanesulfonate (triflate) and a tertiary amine. 106 Use of boron triflates with
a hindered amine favors the Z-enolate. The resulting aldol products are predominantly SECTION 7.7
the syn stereoisomers. The E-boron enolates of some ketones can be preferentially Addition of Enolates and
Enols to Carbonyl
obtained by using dialkylboron chlorides. 107 Compounds: The Aldol
Addition and
Condensation Reactions
(c – C H ) BCl OB(c – C H )
6 11 2
6 11 2
i -Pr NEt
2
O
The contrasting stereoselectivity of the boron triflates and chlorides has been
discussed in terms of reactant conformation and the stereoelectronic requirement for
perpendicular alignment of the hydrogen with the carbonyl group orbital. 108 The
distinction between the two types of borylation reagents seems to lie in the extent
of dissociation of the leaving group. The triflate is likely present as an ion pair,
whereas with the less reactive chloride, the deprotonation may be a concerted (E2-like)
process. The difference between trigonal and tetrahedral coordination of boron affects
the steric interactions and reactant conformation. The two proposed TSs are shown
below.
Cl
R O + BR 2 R + BR
C R O 2 R
H C H C CH 3
CH 3
OBR 2 CH 3 C H OBR 2
H CH 3 H
H
R 3 N:
Z – enolate R N: E – enolate
3
Titanium enolates can be prepared from lithium enolates by reaction with a
trialkoxytitanium(IV) chloride, such as tri-(isopropoxy)titanium chloride. 109 Titanium
enolates can also be prepared directly from ketones by reaction with TiCl and a
4
tertiary amine. 110
OH
O OTiCl 3 O
1) TiCl 4 (CH ) CHCH O
3 2
CH(CH3)2
2) i-Pr NEt
2
CH3
106
D. A. Evans, E. Vogel, and J. V. Nelson, J. Am. Chem. Soc., 101, 6120 (1979); D. A. Evans, J. V. Nelson,
E. Vogel, and T. R. Taber, J. Am. Chem. Soc., 103, 3099 (1981).
107 H. C. Brown, R. K. Dhar, R. K. Bakshi, P. K. Pandiarajan, and B. Singaram, J. Am. Chem. Soc., 111,
3441 (1989); H. C. Brown, R. K. Dhar, K. Ganesan, and B. Singaram, J. Org. Chem., 57, 499 (1992);
H. C. Brown, R. K. Dhar, K.Ganesan, and B. Singaram, J. Org. Chem., 57, 2716 (1992); H. C. Brown,
K. Ganesan, and R. K. Dhar, J. Org. Chem., 58, 147 (1993); K. Ganesan and H. C. Brown, J. Org.
Chem., 58, 7162 (1993).
108
J. M. Goodman and I. Paterson, Tetrahedron Lett., 33, 7223 (1992); E. J. Corey and S. S. Kim, J. Am.
Chem. Soc., 112, 4976 (1990).
109 C. Siegel and E. R. Thornton, J. Am. Chem. Soc., 111, 5722 (1989).
110
D. A. Evans, D. L. Rieger, M. T. Bilodeau, and F. Urpi, J. Am. Chem. Soc., 113, 1047 (1991).