Page 710 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 710
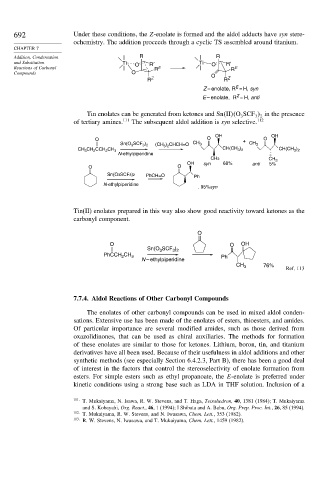
692 Under these conditions, the Z-enolate is formed and the aldol adducts have syn stere-
ochemistry. The addition proceeds through a cyclic TS assembled around titanium.
CHAPTER 7
Addition, Condensation R R
and Substitution Ti O R′ Ti O R′
Reactions of Carbonyl R E R E
Compounds O O
R Z R Z
E
Z – enolate, R = H, syn
Z
E – enolate, R = H, anti
Tin enolates can be generated from ketones and Sn II O SCF in the presence
3
3 2
of tertiary amines. 111 The subsequent aldol addition is syn selective. 112
OH OH
O O + O
(CH 3 ) 2 CHCH=O CH 3 CH 3
Sn(O 3 SCF 3 ) 2
CH 3 CH 2 CCH 2 CH 3 CH(CH 3 ) 2 CH(CH 3 ) 2
N-ethylpiperidine
CH3 CH 3
OH syn 68% anti 5%
O O
Sn(O3SCF3)2 PhCH=O Ph
N-ethylpiperidine
. 95%syn
Tin(II) enolates prepared in this way also show good reactivity toward ketones as the
carbonyl component.
O
O O OH
Sn(O 3 SCF 3 ) 2
PhCCH CH 3 Ph
2
N – ethylpiperidine
CH 76%
3
Ref. 113
7.7.4. Aldol Reactions of Other Carbonyl Compounds
The enolates of other carbonyl compounds can be used in mixed aldol conden-
sations. Extensive use has been made of the enolates of esters, thioesters, and amides.
Of particular importance are several modified amides, such as those derived from
oxazolidinones, that can be used as chiral auxiliaries. The methods for formation
of these enolates are similar to those for ketones. Lithium, boron, tin, and titanium
derivatives have all been used. Because of their usefulness in aldol additions and other
synthetic methods (see especially Section 6.4.2.3, Part B), there has been a good deal
of interest in the factors that control the stereoselectivity of enolate formation from
esters. For simple esters such as ethyl propanoate, the E-enolate is preferred under
kinetic conditions using a strong base such as LDA in THF solution. Inclusion of a
111
T. Mukaiyama, N. Isawa, R. W. Stevens, and T. Haga, Tetrahedron, 40, 1381 (1984); T. Mukaiyama
and S. Kobayahi, Org. React., 46, 1 (1994); I Shibata and A. Babu, Org. Prep. Proc. Int., 26, 85 (1994).
112 T. Mukaiyama, R. W. Stevens, and N. Iwasawa, Chem. Lett., 353 (1982).
113
R. W. Stevens, N. Iwasawa, and T. Mukaiyama, Chem. Lett., 1459 (1982).