Page 708 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 708
690 Under other reaction conditions, the product can result from thermodynamic
control. Aldol reactions can be effected for many compounds using less than a stoichio-
CHAPTER 7 metric amount of base. In these circumstances, the aldol reaction is reversible and the
Addition, Condensation product ratio is determined by the relative stability of the various possible products.
and Substitution
Reactions of Carbonyl Thermodynamic conditions also permit equilibration among all the enolates of the
Compounds nucleophile. The conditions that lead to equilibration include higher reaction temper-
atures, the presence of protic or dissociating polar solvents, and the use of less tightly
coordinating cations.
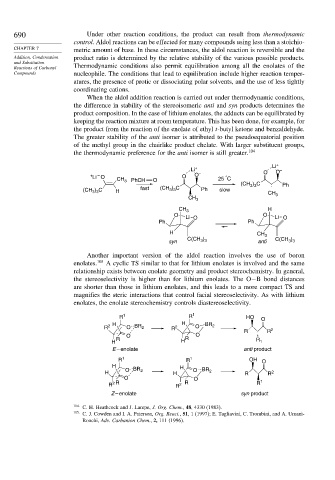
When the aldol addition reaction is carried out under thermodynamic conditions,
the difference in stability of the stereoisomeric anti and syn products determines the
product composition. In the case of lithium enolates, the adducts can be equilibrated by
keeping the reaction mixture at room temperature. This has been done, for example, for
the product from the reaction of the enolate of ethyl t-butyl ketone and benzaldehyde.
The greater stability of the anti isomer is attributed to the pseudoequatorial position
of the methyl group in the chairlike product chelate. With larger substituent groups,
the thermodynamic preference for the anti isomer is still greater. 104
+
Li + O Li O –
+ – O O –
Li O °
CH 3 PhCH O 25 C
(CH ) C Ph
3 3
(CH ) C H fast (CH ) C Ph slow CH
3 3
3 3
CH 3 3
CH 3 H
O O
Li O Li O
Ph Ph
H CH 3
C(CH )
syn 3 3 anti C(CH 3 ) 3
Another important version of the aldol reaction involves the use of boron
enolates. 105 A cyclic TS similar to that for lithium enolates is involved and the same
relationship exists between enolate geometry and product stereochemistry. In general,
the stereoselectivity is higher than for lithium enolates. The O−B bond distances
are shorter than those in lithium enolates, and this leads to a more compact TS and
magnifies the steric interactions that control facial stereoselectivity. As with lithium
enolates, the enolate stereochemistry controls diastereoselectivity.
R 1 R 1 HO
O
2 H BR H BR
R O 2 R 2 O 2
R R 2
O O
R R
H H R 1
E – enolate anti product
R 1 R 1 OH O
H H
H O BR 2 H O BR 2 R R 2
O O
R 2 R R 2 R R 1
Z – enolate syn product
104 C. H. Heathcock and J. Lampe, J. Org. Chem., 48, 4330 (1983).
105
C. J. Cowden and I. A. Paterson, Org. React., 51, 1 (1997); E. Tagliavini, C. Trombini, and A. Umani-
Ronchi, Adv. Carbanion Chem., 2, 111 (1996).