Page 703 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 703
7.7.2. Mixed Aldol Condensations with Aromatic Aldehydes 685
Aldol addition and condensation reactions involving two different carbonyl SECTION 7.7
compounds are called mixed aldol reactions. To be useful as a method for synthesis Addition of Enolates and
there must be some basis for controlling which carbonyl component serves as the Enols to Carbonyl
Compounds: The Aldol
electrophile and which acts as the enolate precursor. One of the most general mixed Addition and
aldol condensations involves the use of aromatic aldehydes with alkyl ketones or Condensation Reactions
aldehydes. There are numerous examples of both acid- and base-catalyzed mixed aldol
condensations involving aromatic aldehydes. The reaction is sometimes referred to as
the Claisen-Schmidt condensation. Aromatic aldehydes are incapable of enolization and
cannot function as the nucleophilic component. Furthermore, dehydration is especially
favorable because the resulting enone is conjugated with the aromatic ring.
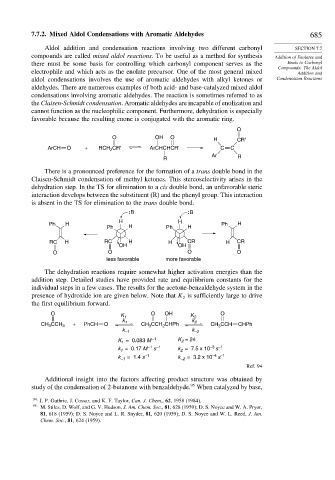
O
O OH O H CR'
ArCH O + RCH CR' ArCHCHCR' C C
2
Ar R
R
There is a pronounced preference for the formation of a trans double bond in the
Claisen-Schmidt condensation of methyl ketones. This stereoselectivity arises in the
dehydration step. In the TS for elimination to a cis double bond, an unfavorable steric
interaction develops between the substituent (R) and the phenyl group. This interaction
is absent in the TS for elimination to the trans double bond.
:B :B
H H
Ph H Ph H
Ph H Ph H
RC H RC H H CR H CR
OH OH
O O O O
less favorable more favorable
The dehydration reactions require somewhat higher activation energies than the
addition step. Detailed studies have provided rate and equilibrium constants for the
individual steps in a few cases. The results for the acetone-benzaldehyde system in the
presence of hydroxide ion are given below. Note that K is sufficiently large to drive
2
the first equilibrium forward.
O K 1 O OH K 2 O
k 1 k 2
CH 3 CCH 3 + PhCH O CH CCH CHPh CH CCH CHPh
3
2
3
k –1 k –2
–1
K = 0.083 M K = 24
2
1
s
s
= 0.17 M –1 –1 k = 7.5 x 10 –3 –1
k 1 2
–1
k –1 = 1.4 s k –2 = 3.2 x 10 –4 –1
s
Ref. 94
Additional insight into the factors affecting product structure was obtained by
95
study of the condensation of 2-butanone with benzaldehyde. When catalyzed by base,
94 J. P. Guthrie, J. Cossar, and K. F. Taylor, Can. J. Chem., 62, 1958 (1984).
95
M. Stiles, D. Wolf, and G. V. Hudson, J. Am. Chem. Soc., 81, 628 (1959); D. S. Noyce and W. A. Pryor,
81, 618 (1959); D. S. Noyce and L. R. Snyder, 81, 620 (1959); D. S. Noyce and W. L. Reed, J. Am.
Chem. Soc., 81, 624 (1959).