Page 699 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 699
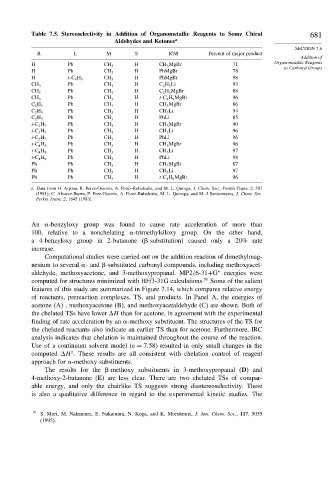
Table 7.5. Stereoselectivity in Addition of Organometallic Reagents to Some Chiral 681
Aldehydes and Ketones a
SECTION 7.6
R L M S R'M Percent of major product
Addition of
H Ph CH 3 H CH 3 MgBr 71 Organometallic Reagents
to Carbonyl Groups
H Ph CH 3 H PhMgBr 78
H t-C 4 H 9 CH 3 H PhMgBr 98
Ph H C 2 H 5 Li 93
CH 3 CH 3
Ph H C 2 H 5 MgBr 88
CH 3 CH 3
Ph H t-C 4 H 9 MgBr 96
CH 3 CH 3
Ph H CH 3 MgBr 86
C 2 H 5 CH 3
Ph H CH 3 Li 94
C 2 H 5 CH 3
Ph H PhLi 85
C 2 H 5 CH 3
Ph H CH 3 MgBr 90
i-C 3 H 7 CH 3
Ph H CH 3 Li 96
i-C 3 H 7 CH 3
Ph H PhLi 96
i-C 3 H 7 CH 3
Ph H CH 3 MgBr 96
t-C 4 H 9 CH 3
Ph H CH 3 Li 97
t-C 4 H 9 CH 3
Ph H PhLi 98
t-C 4 H 9 CH 3
Ph Ph CH 3 H CH 3 MgBr 87
Ph Ph CH 3 H CH 3 Li 97
Ph Ph CH 3 H t-C 4 H 9 MgBr 96
a. Data from O. Arjona, R. Perez-Ossorio, A. Perez-Rubalcaba, and M. L. Quroga, J. Chem. Soc., Perkin Trans. 2, 587
(1981); C. Alvarez-Ibarra, P. Perz-Ossorio, A. Perez-Rubalcaba, M. L. Quiroga, and M. J Santesmases, J. Chem. Soc.
Perkin Trans. 2, 1645 (1983).
An
-benzyloxy group was found to cause rate acceleration of more than
100, relative to a nonchelating
-trimethylsiloxy group. On the other hand,
a 4-benzyloxy group in 2-butanone ( -substitution) caused only a 20% rate
increase.
Computational studies were carried out on the addition reaction of dimethylmag-
nesium to several
- and -substituted carbonyl compounds, including methoxyacet-
aldehyde, methoxyacetone, and 3-methoxypropanal. MP2/6-31+G energies were
∗
91
computed for structures minimized with HF/3-31G calculations. Some of the salient
features of this study are summarized in Figure 7.14, which compares relative energy
of reactants, prereaction complexes, TS, and products. In Panel A, the energies of
acetone (A) , methoxyacetone (B), and methoxyacetaldehyde (C) are shown. Both of
the chelated TSs have lower H than for acetone, in agreement with the experimental
finding of rate acceleration by an
-methoxy substituent. The structures of the TS for
the chelated reactants also indicate an earlier TS than for acetone. Furthermore, IRC
analysis indicates that chelation is maintained throughout the course of the reaction.
Use of a continuum solvent model ( = 7 58) resulted in only small changes in the
‡
computed H . These results are all consistent with chelation control of reagent
approach for
-methoxy substituents.
The results for the -methoxy substituents in 3-methoxypropanal (D) and
4-methoxy-2-butanone (E) are less clear. There are two chelated TSs of compar-
able energy, and only the chairlike TS suggests strong diastereoselectivity. There
is also a qualitative difference in regard to the experimental kinetic studies. The
91
S. Mori, M. Nakamura, E. Nakamura, N. Koga, and K. Morokuma, J. Am. Chem. Soc., 117, 5055
(1995).