Page 698 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 698
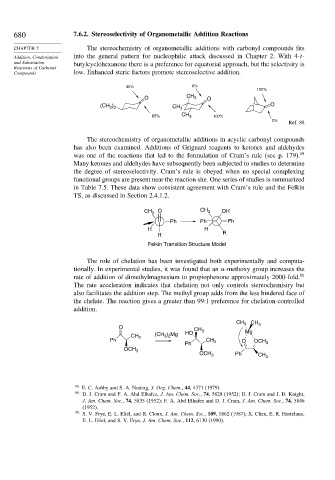
680 7.6.2. Stereoselectivity of Organometallic Addition Reactions
CHAPTER 7 The stereochemistry of organometallic additions with carbonyl compounds fits
Addition, Condensation into the general pattern for nucleophilic attack discussed in Chapter 2. With 4-t-
and Substitution butylcyclohexanone there is a preference for equatorial approach, but the selectivity is
Reactions of Carbonyl
Compounds low. Enhanced steric factors promote stereoselective addition.
35% 0%
100%
O CH 3 O
(CH ) CH 3 O
3 3
CH
65% 3 100%
0%
Ref. 88
The stereochemistry of organometallic additions in acyclic carbonyl compounds
has also been examined. Additions of Grignard reagents to ketones and aldehydes
was one of the reactions that led to the formulation of Cram’s rule (see p. 179). 89
Many ketones and aldehydes have subsequently been subjected to studies to determine
the degree of stereoselectivity. Cram’s rule is obeyed when no special complexing
functional groups are present near the reaction site. One series of studies is summarized
in Table 7.5. These data show consistent agreement with Cram’s rule and the Felkin
TS, as discussed in Section 2.4.1.2.
O CH OH
CH 3 3
Ph Ph Ph
H H
R R
Felkin Transition Structure Model
The role of chelation has been investigated both experimentally and computa-
tionally. In experimental studies, it was found that an
-methoxy group increases the
rate of addition of dimethylmagnesium to propiophenone approximately 2000-fold. 90
The rate acceleration indicates that chelation not only controls stereochemistry but
also facilitates the addition step. The methyl group adds from the less hindered face of
the chelate. The reaction gives a greater than 99:1 preference for chelation-controlled
addition.
CH 3 CH 3
O CH 3 Mg
CH 3 (CH ) Mg HO
3 2
Ph CH 3 O OCH
Ph 3
OCH 3
OCH 3 Ph CH 3
88
E. C. Ashby and S. A. Noding, J. Org. Chem., 44, 4371 (1979).
89 D. J. Cram and F. A. Abd Elhafez, J. Am. Chem. Soc., 74, 5828 (1952); D. J. Cram and J. D. Knight,
J. Am. Chem. Soc., 74, 5835 (1952); F. A. Abd Elhafez and D. J. Cram, J. Am. Chem. Soc., 74, 5846
(1952).
90
S. V. Frye, E. L. Eliel, and R. Cloux, J. Am. Chem. Soc., 109, 1862 (1987); X. Chen, E. R. Hortelano,
E. L. Eliel, and S. V. Frye, J. Am. Chem. Soc., 112, 6130 (1990).