Page 700 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 700
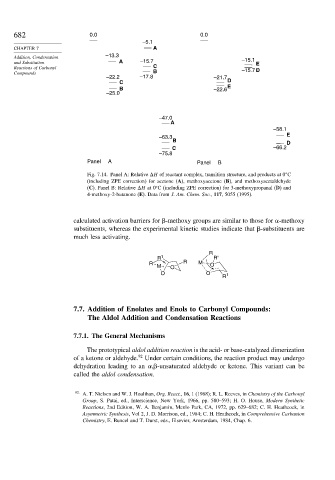
682 0.0 0.0
–5.1
CHAPTER 7 A
Addition, Condensation –13.3 –15.1
and Substitution A –15.7 E
Reactions of Carbonyl C –15.7 D
Compounds B
–22.2 –17.8 –21.7
C D
B –22.6 E
–25.0
–47.0
A
–58.1
–63.3 E
B D
C –66.2
–75.8
Panel A Panel B
Fig. 7.14. Panel A: Relative H of reactant complex, transition structure, and products at 0 C
(including ZPE correction) for acetone (A), methoxyacetone (B), and methoxyacetaldehyde
(C). Panel B: Relative H at 0 C (including ZPE correction) for 3-methoxypropanal (D) and
4-methoxy-2-butanone (E). Data from J. Am. Chem. Soc., 117, 5055 (1995).
calculated activation barriers for -methoxy groups are similar to those for
-methoxy
substituents, whereas the experimental kinetic studies indicate that -substituents are
much less activating.
R
R 1 R'
R R M O
M O
O O 1
R
7.7. Addition of Enolates and Enols to Carbonyl Compounds:
The Aldol Addition and Condensation Reactions
7.7.1. The General Mechanisms
The prototypical aldol addition reaction is the acid- or base-catalyzed dimerization
92
of a ketone or aldehyde. Under certain conditions, the reaction product may undergo
dehydration leading to an , -unsaturated aldehyde or ketone. This variant can be
called the aldol condensation.
92
A. T. Nielsen and W. J. Houlihan, Org. React., 16, 1 (1968); R. L. Reeves, in Chemistry of the Carbonyl
Group, S. Patai, ed., Interscience, New York, 1966, pp. 580–593; H. O. House, Modern Synthetic
Reactions, 2nd Edition, W. A. Benjamin, Menlo Park, CA, 1972, pp. 629–682; C. H. Heathcock, in
Asymmetric Synthesis, Vol 2, J. D. Morrison, ed., 1984; C. H. Heathcock, in Comprehensive Carbanion
Chemistry, E. Buncel and T. Durst, eds., Elsevier, Amsterdam, 1984, Chap. 6.