Page 695 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 695
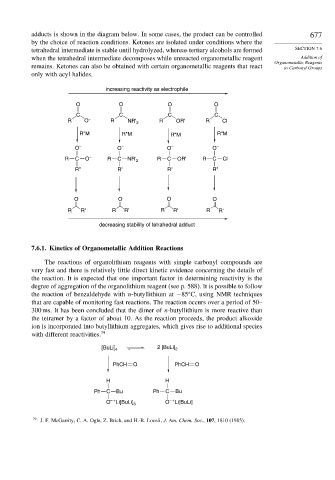
adducts is shown in the diagram below. In some cases, the product can be controlled 677
by the choice of reaction conditions. Ketones are isolated under conditions where the
tetrahedral intermediate is stable until hydrolyzed, whereas tertiary alcohols are formed SECTION 7.6
when the tetrahedral intermediate decomposes while unreacted organometallic reagent Addition of
Organometallic Reagents
remains. Ketones can also be obtained with certain organometallic reagents that react to Carbonyl Groups
only with acyl halides.
increasing reactivity as electrophile
O O O O
C C C C
R O – R NR' 2 R OR' R Cl
R"M R"M R"M R"M
O – O – O – O –
R C O – R C NR' 2 R C OR' R C Cl
R" R" R" R"
O O O O
R R' R R' R R' R R'
decreasing stability of tetrahedral adduct
7.6.1. Kinetics of Organometallic Addition Reactions
The reactions of organolithium reagents with simple carbonyl compounds are
very fast and there is relatively little direct kinetic evidence concerning the details of
the reaction. It is expected that one important factor in determining reactivity is the
degree of aggregation of the organolithium reagent (see p. 588). It is possible to follow
the reaction of benzaldehyde with n-butyllithium at −85 C, using NMR techniques
that are capable of monitoring fast reactions. The reaction occurs over a period of 50–
300 ms. It has been concluded that the dimer of n-butyllithium is more reactive than
the tetramer by a factor of about 10. As the reaction proceeds, the product alkoxide
ion is incorporated into butyllithium aggregates, which gives rise to additional species
with different reactivities. 79
[BuLi] 4 2 [BuLi] 2
PhCH O PhCH O
H H
Ph C Bu Ph C Bu
– +
– +
O Li[BuLi] 3 O Li[BuLi]
79
J. F. McGarrity, C. A. Ogle, Z. Brich, and H.-R. Loosli, J. Am. Chem. Soc., 107, 1810 (1985).