Page 690 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 690
672 the histidine residue in proteins is involved in enzyme-catalyzed hydrolyses. Secondly,
the imidazole ring has several possible catalytic functions, which include acting as a
CHAPTER 7 general acid in the protonated form, acting as a general base, or acting as a nucleophile
Addition, Condensation in the neutral form. A study of a number of derivatives of Structure 3 was undertaken
and Substitution 71
Reactions of Carbonyl to distinguish between these various possible mechanisms as a function of pH.
Compounds
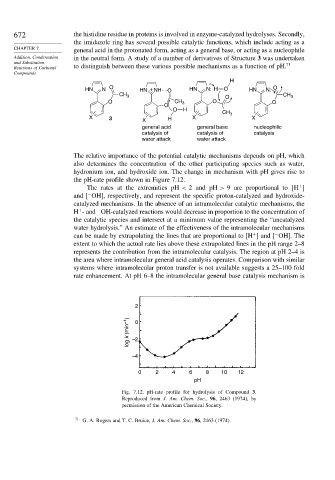
H
HN N O HN + NH O HN N: H O HN N: O
CH 3 O CH 3
O CH 3 O O
O
O H
CH 3
X 3 X H X X
general acid general base nucleophilic
catalysis of catalysis of catalysis
water attack water attack
The relative importance of the potential catalytic mechanisms depends on pH, which
also determines the concentration of the other participating species such as water,
hydronium ion, and hydroxide ion. The change in mechanism with pH gives rise to
the pH-rate profile shown in Figure 7.12.
+
The rates at the extremities pH < 2 and pH > 9 are proportional to [H ]
and [ OH], respectively, and represent the specific proton-catalyzed and hydroxide-
−
catalyzed mechanisms. In the absence of an intramolecular catalytic mechanisms, the
−
H - and OH-catalyzed reactions would decrease in proportion to the concentration of
+
the catalytic species and intersect at a minimum value representing the “uncatalyzed
water hydrolysis.” An estimate of the effectiveness of the intramolecular mechanisms
can be made by extrapolating the lines that are proportional to [H ] and [ OH]. The
−
+
extent to which the actual rate lies above these extrapolated lines in the pH range 2–8
represents the contribution from the intramolecular catalysis. The region at pH 2–4 is
the area where intramolecular general acid catalysis operates. Comparison with similar
systems where intramolecular proton transfer is not available suggests a 25–100 fold
rate enhancement. At pH 6–8 the intramolecular general base catalysis mechanism is
2
log k (min –1 ) –2 0
–4
0 2 4 6 8 10 12
pH
Fig. 7.12. pH-rate profile for hydrolysis of Compound 3.
Reproduced from J. Am. Chem. Soc., 96, 2463 (1974), by
permission of the American Chemical Society.
71
G. A. Rogers and T. C. Bruice, J. Am. Chem. Soc., 96, 2463 (1974).