Page 688 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 688
670
40
CHAPTER 7
Addition, Condensation
and Substitution
Reactions of Carbonyl 30
k obsd × 103 sec –1 20
Compounds
10
0
1 2 3 4 5 6 7
pH
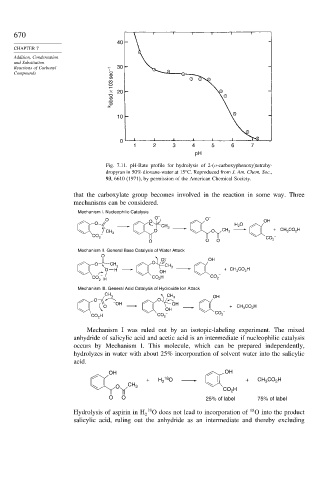
Fig. 7.11. pH-Rate profile for hydrolysis of 2-(o-carboxyphenoxy)tetrahy-
dropyran in 50% dioxane-water at 15 C. Reproduced from J. Am. Chem. Soc.,
93, 6610 (1971), by permission of the American Chemical Society.
that the carboxylate group becomes involved in the reaction in some way. Three
mechanisms can be considered.
Mechanism I. Nucleophilic Catalysis
O – –
O O OH
O O H O
CH 3 2
O O CH + CH CO H
CH 3 3 3 2
CO 2 – CO –
O O O 2
Mechanism II. General Base Catalysis of Water Attack
O
O – OH
O CH O CH
3 3
O H + CH 3 CO H
OH 2
– –
CO CO H CO 2
2 H 2
Mechanism III. General Acid Catalysis of Hydroxide Ion Attack
CH
3 CH 3 OH
O O
– OH OH
O + CH CO H
OH 3 2
– CO 2 –
CO H CO 2
2
Mechanism I was ruled out by an isotopic-labeling experiment. The mixed
anhydride of salicylic acid and acetic acid is an intermediate if nucleophilic catalysis
occurs by Mechanism I. This molecule, which can be prepared independently,
hydrolyzes in water with about 25% incorporation of solvent water into the salicylic
acid.
OH OH
+ H 2 18 O + CH CO H
2
3
O CH 3
CO 2 H
O O 25% of label 75% of label
Hydrolysis of aspirin in H 18 O does not lead to incorporation of 18 O into the product
2
salicylic acid, ruling out the anhydride as an intermediate and thereby excluding