Page 692 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 692
674
–1.0
CHAPTER 7
(b)
Addition, Condensation –2.0
and Substitution –2.0
Log k obsd (S –1 ) –4.0 log 10 (k obs /s –1 ) –3.0
Reactions of Carbonyl
Compounds –3.0 (a)
–5.0
–4.0
2 3 4 5 6 7 8 9 10 11
pH
–5.0
3.0 4.0 5.0 6.0 7.0 8.0 9.0 10.0 11.0 12.0 13.0
pH
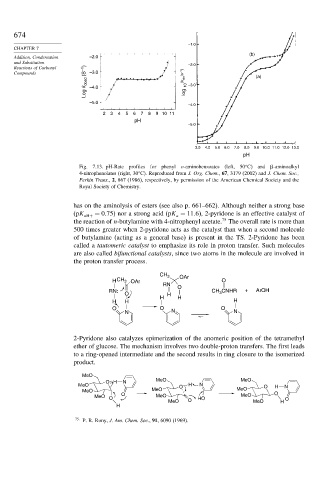
Fig. 7.13. pH-Rate profiles for phenyl o-aminobenzoates (left, 50 C) and -aminoalkyl
4-nitrophenolates (right, 30 C). Reproduced from J. Org. Chem., 67, 3179 (2002) and J. Chem. Soc.,
Perkin Trans., 2, 867 (1986), respectively, by permission of the American Chemical Society and the
Royal Society of Chemistry.
has on the aminolysis of esters (see also p. 661–662). Although neither a strong base
(pK = 0 75) nor a strong acid (pK = 11 6), 2-pyridone is an effective catalyst of
aH+ a
75
the reaction of n-butylamine with 4-nitrophenyl acetate. The overall rate is more than
500 times greater when 2-pyridone acts as the catalyst than when a second molecule
of butylamine (acting as a general base) is present in the TS. 2-Pyridone has been
called a tautomeric catalyst to emphasize its role in proton transfer. Such molecules
are also called bifunctional catalysts, since two atoms in the molecule are involved in
the proton transfer process.
CH 3 OAr
H CH 3 OAr O
RN
O
RN: CH CNHR + ArOH
O H 3
H H
H H H
O O O
N N N
2-Pyridone also catalyzes epimerization of the anomeric position of the tetramethyl
ether of glucose. The mechanism involves two double-proton transfers. The first leads
to a ring-opened intermediate and the second results in ring closure to the isomerized
product.
MeO
MeO MeO
O: H N
MeO O H N O H N
MeO MeO MeO O
MeO O O MeO HO MeO
MeO O MeO H O
H
75
P. R. Rony, J. Am. Chem. Soc., 91, 6090 (1969).