Page 689 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 689
Mechanism I. 68 Mechanism III cannot be distinguished from the first two on the 671
basis of kinetics alone, because the reactive species shown is in rapid equilibrium
with the anion and therefore equivalent to it in terms of reaction kinetics. The SECTION 7.5
general acid catalysis of Mechanism III can be eliminated on the basis of failure Intramolecular Catalysis
of Carbonyl Substitution
of other nucleophiles to show evidence for general acid catalysis by the neigh- Reactions
boring carboxylic acid group. Since there is no reason to believe hydroxide should
be special in this way, Mechanism III is ruled out. Thus Mechanism II, general base
catalysis of water attack, is believed to be the correct description of the hydrolysis of
aspirin.
The extent to which intramolecular nucleophilic catalysis of the type depicted in
Mechanism I is important is a function of the leaving ability of the alkoxy group. This
has been demonstrated by the study of the hydrolysis of a series of monoesters of
phthalic acid.
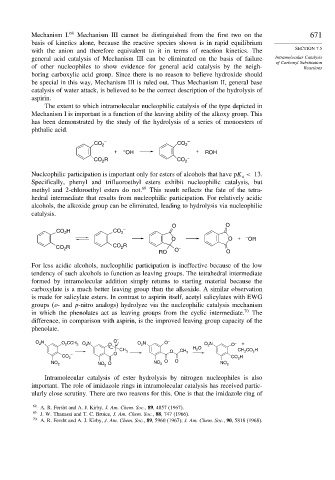
CO 2 – CO 2 –
+ – OH + ROH
CO R CO 2 –
2
Nucleophilic participation is important only for esters of alcohols that have pK < 13.
a
Specifically, phenyl and trifluoroethyl esters exhibit nucleophilic catalysis, but
methyl and 2-chloroethyl esters do not. 69 This result reflects the fate of the tetra-
hedral intermediate that results from nucleophilic participation. For relatively acidic
alcohols, the alkoxide group can be eliminated, leading to hydrolysis via nucleophilic
catalysis.
O O
CO H CO 2 –
2
O O + – OR
R CO R
CO 2 2 O –
RO O
For less acidic alcohols, nucleophilic participation is ineffective because of the low
tendency of such alcohols to function as leaving groups. The tetrahedral intermediate
formed by intramolecular addition simply returns to starting material because the
carboxylate is a much better leaving group than the alkoxide. A similar observation
is made for salicylate esters. In contrast to aspirin itself, acetyl salicylates with EWG
groups (o- and p-nitro analogs) hydrolyze via the nucleophilic catalysis mechanism
in which the phenolates act as leaving groups from the cyclic intermediate. 70 The
difference, in comparison with aspirin, is the improved leaving group capacity of the
phenolate.
O 2 N O 2 CCH 3 O 2 N O – O 2 N O – +
O O 2 N O –
H 2 O
CH 3 O CH 3 CH 3 CO 2 H
– O
CO 2 CO 2 H
O O
O NO 2
NO 2 NO 2
NO 2
Intramolecular catalysis of ester hydrolysis by nitrogen nucleophiles is also
important. The role of imidazole rings in intramolecular catalysis has received partic-
ularly close scrutiny. There are two reasons for this. One is that the imidazole ring of
68 A. R. Fersht and A. J. Kirby, J. Am. Chem. Soc., 89, 4857 (1967).
69 J. W. Thanassi and T. C. Bruice, J. Am. Chem. Soc., 88, 747 (1966).
70
A. R. Fersht and A. J. Kirby, J. Am. Chem. Soc., 89, 5960 (1967); J. Am. Chem. Soc., 90, 5818 (1968).