Page 693 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 693
Other compounds such as benzoic acid and pyrazole, which can effect similar concerted 675
proton transfer and avoid charged species, also catalyze this and related reactions. 76
Another type of bifunctional catalysis has been noted with
-diamines in which SECTION 7.5
one of the amino groups is primary and the other tertiary. These substituted diamines are Intramolecular Catalysis
of Carbonyl Substitution
from several times to as much as 1000 times more reactive toward imine formation than Reactions
77
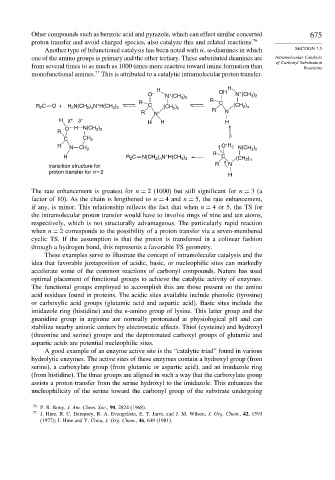
monofunctional amines. This is attributed to a catalytic intramolecular proton transfer.
H H
+
O – N (CH ) OH N (CH )
+
3 2
3 2
+
R 2 C O + H 2 N(CH 2 ) n N H(CH 3 ) 2 R C (CH ) R C (CH )
2 n
2 n
R N + R N
H δ + δ + H H H
O H N(CH )
3 2
R
C CH 2
+
R N CH 2 O H 2 N(CH )
3 2
R
) N H(CH )
H R C N(CH 2 n + 3 2 C (CH 2 ) n
2
transition structure for R N
proton transfer for n = 2
H
The rate enhancement is greatest for n = 2 (1000) but still significant for n = 3(a
factor of 10). As the chain is lengthened to n = 4 and n = 5, the rate enhancement,
if any, is minor. This relationship reflects the fact that when n = 4 or 5, the TS for
the intramolecular proton transfer would have to involve rings of nine and ten atoms,
respectively, which is not structurally advantageous. The particularly rapid reaction
when n = 2 corresponds to the possibility of a proton transfer via a seven-membered
cyclic TS. If the assumption is that the proton is transferred in a colinear fashion
through a hydrogen bond, this represents a favorable TS geometry.
These examples serve to illustrate the concept of intramolecular catalysis and the
idea that favorable juxtaposition of acidic, basic, or nucleophilic sites can markedly
accelerate some of the common reactions of carbonyl compounds. Nature has used
optimal placement of functional groups to achieve the catalytic activity of enzymes.
The functional groups employed to accomplish this are those present on the amino
acid residues found in proteins. The acidic sites available include phenolic (tyrosine)
or carboxylic acid groups (glutamic acid and aspartic acid). Basic sites include the
imidazole ring (histidine) and the -amino group of lysine. This latter group and the
guanidine group in arginine are normally protonated at physiological pH and can
stabilize nearby anionic centers by electrostatic effects. Thiol (cysteine) and hydroxyl
(threonine and serine) groups and the deprotonated carboxyl groups of glutamic and
aspartic acids are potential nucleophilic sites.
A good example of an enzyme active site is the “catalytic triad” found in various
hydrolytic enzymes. The active sites of these enzymes contain a hydroxyl group (from
serine), a carboxylate group (from glutamic or aspartic acid), and an imidazole ring
(from histidine). The three groups are aligned in such a way that the carboxylate group
assists a proton transfer from the serine hydroxyl to the imidazole. This enhances the
nucleophilicity of the serine toward the carbonyl group of the substrate undergoing
76 P. R. Rony, J. Am. Chem. Soc., 90, 2824 (1968).
77
J. Hine, R. C. Dempsey, R. A. Evangelista, E. T. Jarvi, and J. M. Wilson, J. Org. Chem., 42, 1593
(1977); J. Hine and Y. Chou, J. Org. Chem., 46, 649 (1981).