Page 702 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 702
684 In general, the reactions in the addition phase of both the base- and acid-catalyzed
mechanisms are reversible. The equilibrium constant for addition is usually unfavorable
CHAPTER 7 for ketones. The equilibrium constant for the dehydration phase is usually favorable
Addition, Condensation because of the conjugated
,ß-unsaturated carbonyl system that is formed. When
and Substitution
Reactions of Carbonyl the reaction conditions are sufficiently vigorous to cause dehydration, the overall
Compounds reaction can go to completion, even if the equilibrium constant for the addition step is
unfavorable.
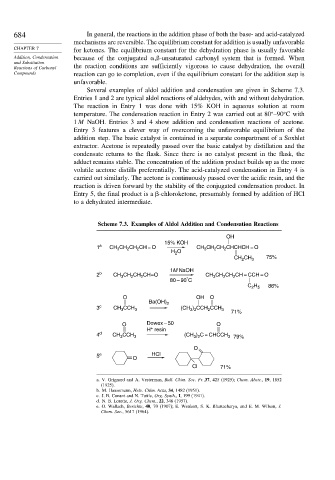
Several examples of aldol addition and condensation are given in Scheme 7.3.
Entries 1 and 2 are typical aldol reactions of aldehydes, with and without dehydration.
The reaction in Entry 1 was done with 15% KOH in aqueous solution at room
temperature. The condensation reaction in Entry 2 was carried out at 80 –90 C with
1M NaOH. Entries 3 and 4 show addition and condensation reactions of acetone.
Entry 3 features a clever way of overcoming the unfavorable equilibrium of the
addition step. The basic catalyst is contained in a separate compartment of a Soxhlet
extractor. Acetone is repeatedly passed over the basic catalyst by distillation and the
condensate returns to the flask. Since there is no catalyst present in the flask, the
adduct remains stable. The concentration of the addition product builds up as the more
volatile acetone distills preferentially. The acid-catalyzed condensation in Entry 4 is
carried out similarly. The acetone is continuously passed over the acidic resin, and the
reaction is driven forward by the stability of the conjugated condensation product. In
Entry 5, the final product is a -chloroketone, presumably formed by addition of HCl
to a dehydrated intermediate.
Scheme 7.3. Examples of Aldol Addition and Condensation Reactions
OH
15% KOH
1 a CH CH CH CH = O CH 3 CH CH CHCHCH = O
2
3
2
2
2
H O
2
CH CH 3 75%
2
1M NaOH
2 b CH CH CH CH=O CH CH CH CH = CCH = O
2
2
2
3
3
2
°
80 – 90 C
H 86%
C 2 5
O OH O
Ba(OH) 2
3 c CH CCH 3 (CH ) CCH CCH 3
3 2
2
3
71%
O Dowex – 50 O
+
H resin
4 d CH CCH 3 (CH ) C = CHCCH 3 79%
3 2
3
O
5 e HCl
O
Cl 71%
a. V. Grignard and A. Vesterman, Bull. Chim. Soc. Fr.,37, 425 (1925); Chem. Abstr., 19, 1852
(1925).
b. M. Hausemann, Helv. Chim. Acta, 34, 1482 (1951).
c. J. B. Conant and N. Tuttle, Org. Synth., I, 199 (1941).
d. N. B. Lorette, J. Org. Chem., 22, 346 (1957).
e. O. Wallach, Berichte, 40, 70 (1907); E. Wenkert, S. K. Bhattacharya, and E. M. Wilson, J.
Chem. Soc., 5617 (1964).