Page 706 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 706
688 as the nucleophile. This can be achieved by preforming the reactive nucleophilic
enolate and ensuring that the addition step is fast relative to proton exchange between
CHAPTER 7
the nucleophilic and electrophilic reactants. These reactions are under kinetic control,
Addition, Condensation both at the stage of forming the enolate and at the addition step. The enolate that is
and Substitution
Reactions of Carbonyl to serve as the nucleophile is generated stoichiometrically, usually with lithium as the
Compounds
counterion in an aprotic solvent. Under these conditions enolates do not equilibrate
with the other regio- or stereoisomeric enolates that can be formed from the ketone (see
Section 6.3). The electrophilic carbonyl compound is then added. The structure of the
reaction product is determined primarily by two factors: (1) the E-or Z-configuration
of the initial enolate and (2) the structure of the TS for addition to the electrophilic
carbonyl group.
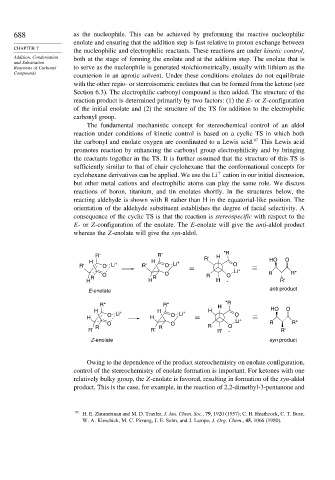
The fundamental mechanistic concept for stereochemical control of an aldol
reaction under conditions of kinetic control is based on a cyclic TS in which both
the carbonyl and enolate oxygen are coordinated to a Lewis acid. 97 This Lewis acid
promotes reaction by enhancing the carbonyl group electrophilicity and by bringing
the reactants together in the TS. It is further assumed that the structure of this TS is
sufficiently similar to that of chair cyclohexane that the conformational concepts for
+
cyclohexane derivatives can be applied. We use the Li cation in our initial discussion,
but other metal cations and electrophilic atoms can play the same role. We discuss
reactions of boron, titanium, and tin enolates shortly. In the structures below, the
reacting aldehyde is shown with R rather than H in the equatorial-like position. The
orientation of the aldehyde substituent establishes the degree of facial selectivity. A
consequence of the cyclic TS is that the reaction is stereospecific with respect to the
E-or Z-configuration of the enolate. The E-enolate will give the anti-aldol product
whereas the Z-enolate will give the syn-aldol.
R" R" R' H "R
H + H + HO O
R' O Li R' O Li O
Li + R R"
O O R O
R R
H H H - R'
E-enolate anti product
R" R" H "R
H + H + H HO O
O Li O Li O
H H +
O O Li R R"
R R R O
R' R' R' - R'
Z-enolate syn product
Owing to the dependence of the product stereochemistry on enolate configuration,
control of the stereochemistry of enolate formation is important. For ketones with one
relatively bulky group, the Z-enolate is favored, resulting in formation of the syn-aldol
product. This is the case, for example, in the reaction of 2,2-dimethyl-3-pentanone and
97
H. E. Zimmerman and M. D. Traxler, J. Am. Chem. Soc., 79, 1920 (1957); C. H. Heathcock, C. T. Buse,
W. A. Kleschick, M. C. Pirrung, J. E. Sohn, and J. Lampe, J. Org. Chem., 45, 1066 (1980).