Page 704 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 704
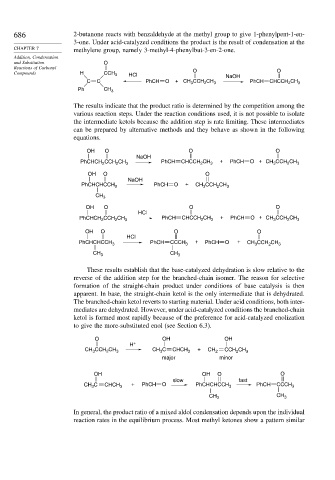
686 2-butanone reacts with benzaldehyde at the methyl group to give 1-phenylpent-1-en-
3-one. Under acid-catalyzed conditions the product is the result of condensation at the
CHAPTER 7 methylene group, namely 3-methyl-4-phenylbut-3-en-2-one.
Addition, Condensation
and Substitution O
Reactions of Carbonyl
Compounds H CCH 3 HCl O NaOH O
C C PhCH O + CH 3 CCH 2 CH 3 PhCH CHCCH 2 CH 3
Ph CH 3
The results indicate that the product ratio is determined by the competition among the
various reaction steps. Under the reaction conditions used, it is not possible to isolate
the intermediate ketols because the addition step is rate limiting. These intermediates
can be prepared by alternative methods and they behave as shown in the following
equations.
OH O O O
NaOH
PhCHCH CCH CH 3 PhCH CHCCH CH 3 + PhCH O + CH CCH CH 3
2
2
2
3
2
OH O O
NaOH
2
PhCHCHCCH 3 PhCH O + CH CCH CH 3
3
CH 3
OH O O O
HCl
PhCHCH 2 CCH CH 3 PhCH CHCCH 2 CH 3 + PhCH O + CH CCH CH 3
3
2
2
OH O O O
HCl
PhCHCHCCH 3 PhCH CCCH 3 + PhCH O + CH CCH CH 3
3
2
CH 3 CH 3
These results establish that the base-catalyzed dehydration is slow relative to the
reverse of the addition step for the branched-chain isomer. The reason for selective
formation of the straight-chain product under conditions of base catalysis is then
apparent. In base, the straight-chain ketol is the only intermediate that is dehydrated.
The branched-chain ketol reverts to starting material. Under acid conditions, both inter-
mediates are dehydrated. However, under acid-catalyzed conditions the branched-chain
ketol is formed most rapidly because of the preference for acid-catalyzed enolization
to give the more-substituted enol (see Section 6.3).
O OH OH
H +
CH CCH CH 3 CH C CHCH 3 + CH 2 CCH CH 3
2
3
2
3
major minor
OH OH O O
slow fast
CH C CHCH 3 + PhCH O PhCHCHCCH 3 PhCH CCCH 3
3
CH
CH 3 3
In general, the product ratio of a mixed aldol condensation depends upon the individual
reaction rates in the equilibrium process. Most methyl ketones show a pattern similar