Page 707 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 707
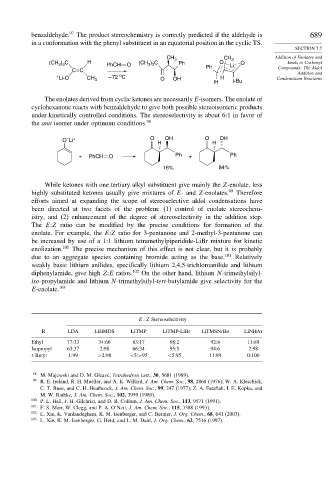
benzaldehyde. 97 The product stereochemistry is correctly predicted if the aldehyde is 689
in a conformation with the phenyl substituent in an equatorial position in the cyclic TS.
SECTION 7.7
CH 3 CH 3 Addition of Enolates and
) C
(CH ) C H PhCH O (CH 3 3 Ph O Li O Enols to Carbonyl
3 3
CC Ph Compounds: The Aldol
Addition and
o
+ – 72 C
Li-O CH 3 O OH H Condensation Reactions
H t-Bu
The enolates derived from cyclic ketones are necessarily E-isomers. The enolate of
cyclohexanone reacts with benzaldehyde to give both possible stereoisomeric products
under kinetically controlled conditions. The stereoselectivity is about 6:1 in favor of
the anti isomer under optimum conditions. 98
–
O Li + O OH O OH
H H
+ PhCH O Ph + Ph
16% 84%
While ketones with one tertiary alkyl substituent give mainly the Z-enolate, less
highly substituted ketones usually give mixtures of E- and Z-enolates. 99 Therefore
efforts aimed at expanding the scope of stereoselective aldol condensations have
been directed at two facets of the problem: (1) control of enolate stereochem-
istry, and (2) enhancement of the degree of stereoselectivity in the addition step.
The E:Z ratio can be modified by the precise conditions for formation of the
enolate. For example, the E:Z ratio for 3-pentanone and 2-methyl-3-pentanone can
be increased by use of a 1:1 lithium tetramethylpiperidide-LiBr mixture for kinetic
enolization. 100 The precise mechanism of this effect is not clear, but it is probably
due to an aggregate species containing bromide acting as the base. 101 Relatively
weakly basic lithium anilides, specifically lithium 2,4,5-trichloroanilide and lithium
diphenylamide, give high Z:E ratios. 102 On the other hand, lithium N-trimethylsilyl-
iso-propylamide and lithium N-trimethylsilyl-tert-butylamide give selectivity for the
E-enolate. 103
E Z Stereoselectivity
R LDA LiHMDS LiTMP LiTMP-LiBr LiTMSNtBu LiNHAr
Ethyl 77:33 34:66 83:17 98:2 92:8 11:89
Isopropyl 63:37 2:98 66:34 95:5 94:6 2:98
t-Butyl 1:99 >2 98 <5 >95 <5 95 11:89 0:100
98
M. Majewski and D. M. Gleave, Tetrahedron Lett., 30, 5681 (1989).
99 R. E. Ireland, R. H. Mueller, and A. K. Willard, J. Am. Chem. Soc., 98, 2868 (1976); W. A. Kleschick,
C. T. Buse, and C. H. Heathcock, J. Am. Chem. Soc., 99, 247 (1977); Z. A. Fataftah, I. E. Kopka, and
M. W. Rathke, J. Am. Chem. Soc., 102, 3959 (1980).
100
P. L. Hall, J. H. Gilchrist, and D. B. Collum, J. Am. Chem. Soc., 113, 9571 (1991).
101
F. S. Mair, W. Clegg, and P. A. O’Neil, J. Am. Chem. Soc., 115, 3388 (1993).
102 L. Xie, K. Vanlandeghem, K. M. Isenberger, and C. Bernier, J. Org. Chem., 68, 641 (2003).
103
L. Xie, K. M. Isenberger, G. Held, and L. M. Dahl, J. Org. Chem., 62, 7516 (1997).