Page 712 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 712
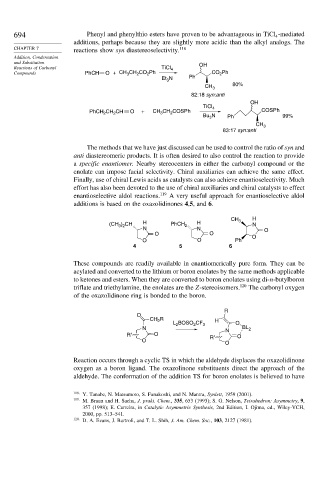
694 Phenyl and phenylthio esters have proven to be advantageous in TiCl -mediated
4
additions, perhaps because they are slightly more acidic than the alkyl analogs. The
CHAPTER 7 reactions show syn diastereoselectivity. 118
Addition, Condensation
and Substitution OH
Reactions of Carbonyl TiCl 4
Compounds PhCH O + CH CH CO Ph CO Ph
2
2
3
2
Et N Ph
3
CH 3 80%
82:18 syn:anti
OH
TiCl 4
CH CH O + CH CH COSPh COSPh
PhCH 2 2 3 2
Bu N Ph 99%
3
CH 3
83:17 syn:anti
The methods that we have just discussed can be used to control the ratio of syn and
anti diastereomeric products. It is often desired to also control the reaction to provide
a specific enantiomer. Nearby stereocenters in either the carbonyl compound or the
enolate can impose facial selectivity. Chiral auxiliaries can achieve the same effect.
Finally, use of chiral Lewis acids as catalysts can also achieve enantioselectivity. Much
effort has also been devoted to the use of chiral auxiliaries and chiral catalysts to effect
enantioselective aldol reactions. 119 A very useful approach for enantioselective aldol
additions is based on the oxazolidinones 4,5, and 6.
CH 3 H
(CH ) CH H PhCH 2 H N
3 2
N N O
O O
O O Ph O
4 5 6
These compounds are readily available in enantiomerically pure form. They can be
acylated and converted to the lithium or boron enolates by the same methods applicable
to ketones and esters. When they are converted to boron enolates using di-n-butylboron
triflate and triethylamine, the enolates are the Z-stereoisomers. 120 The carbonyl oxygen
of the oxazolidinone ring is bonded to the boron.
R
O
CH 2 R H
2
L 2 BOSO CF 3 O
N N BL 2
R' O R' O
O
O
Reaction occurs through a cyclic TS in which the aldehyde displaces the oxazolidinone
oxygen as a boron ligand. The oxazolinone substituents direct the approach of the
aldehyde. The conformation of the addition TS for boron enolates is believed to have
118
Y. Tanabe, N. Matsumoto, S. Funakoshi, and N. Mantra, Synlett, 1959 (2001).
119 M. Braun and H. Sacha, J. prakt. Chem., 335, 653 (1993); S. G. Nelson, Tetrahedron: Asymmetry, 9,
357 (1998); E. Carreira, in Catalytic Asymmetric Synthesis, 2nd Edition, I. Ojima, ed., Wiley-VCH,
2000, pp. 513–541.
120
D. A. Evans, J. Bartroli, and T. L. Shih, J. Am. Chem. Soc., 103, 2127 (1981).