Page 716 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 716
698 General References
CHAPTER 7
M. L. Bender, Mechanisms of Homogeneous Catalysis from Protons to Proteins, Wiley-Interscience, New
Addition, Condensation York, 1971.
and Substitution T. C. Bruice and S. J. Benkovic, Bioorganic Mechanisms, W. A. Benjamin, New York, 1966.
Reactions of Carbonyl H. Dugas and C. Penney, Bioorganic Chemistry: A Chemical Approach to Enzyme Action, 3rd Edition,
Compounds
Springer-Verlag, New York, 1996.
W. P. Jencks, Catalysis in Chemistry and Enzymology, McGraw-Hill, New York, 1969.
A. J. Kirby and A. R. Fersht, Progress in Bioorganic Chemistry, Vol. 1, E. T. Kaiser and R. J. Kezdy, eds.,
Wiley-Interscience, New York, 1971, pp. 1–82.
S. Patai, ed., The Chemistry of Acid Derivatives, Suppl. B, Vol. 2, Wiley, New York, 1992.
S. Patai, ed., The Chemistry of the Carbonyl Group, Wiley-Interscience, New York, 1969.
S. Patai, ed., The Chemistry of Carboxylic Acids and Esters, Wiley-Interscience, New York, 1969.
J. E. Zabricky,ed., The Chemistry of Amides, Wiley- Interscience, New York, 1970.
Problems
(References for these problems will be found on page 1162.)
7.1. The hydrates of aldehydes and ketones are considerably more acidic than
alcohols (pK 16–19). Some values are shown below. How do you account for
this enhanced acidity? Explain the relative order of acidity for the compounds
in the list.
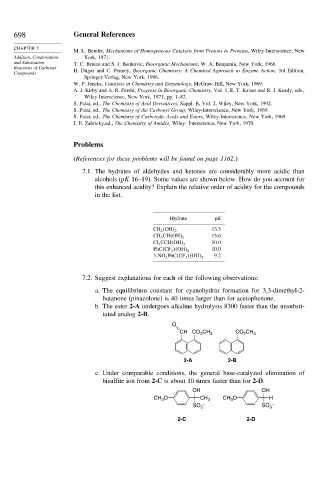
Hydrate pK
13.3
CH 2 OH 2
13.6
CH 3 CH OH 2
10.0
Cl 3 CCH OH 2
10.0
PhC CF 3 OH 2
9.2
3-NO 2 PhC CF 3 OH 2
7.2. Suggest explanations for each of the following observations:
a. The equilibrium constant for cyanohydrin formation for 3,3-dimethyl-2-
butanone (pinacolone) is 40 times larger than for acetophenone.
b. The ester 2-A undergoes alkaline hydrolysis 8300 faster than the unsubsti-
tuted analog 2-B.
O
CH CO CH 3 CO CH 3
2
2
2-A 2-B
c. Under comparable conditions, the general base-catalyzed elimination of
bisulfite ion from 2-C is about 10 times faster than for 2-D.
OH OH
CH O CH 3 CH O H
3
3
– –
SO 3 SO 3
2-C 2-D