Page 705 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 705
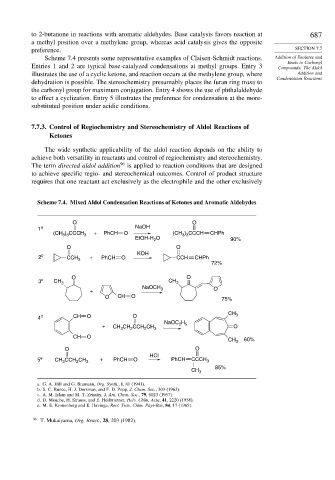
to 2-butanone in reactions with aromatic aldehydes. Base catalysis favors reaction at 687
a methyl position over a methylene group, whereas acid catalysis gives the opposite
preference. SECTION 7.7
Scheme 7.4 presents some representative examples of Claisen-Schmidt reactions. Addition of Enolates and
Enols to Carbonyl
Entries 1 and 2 are typical base-catalyzed condensations at methyl groups. Entry 3 Compounds: The Aldol
illustrates the use of a cyclic ketone, and reaction occurs at the methylene group, where Addition and
Condensation Reactions
dehydration is possible. The stereochemistry presumably places the furan ring trans to
the carbonyl group for maximum conjugation. Entry 4 shows the use of phthalaldehyde
to effect a cyclization. Entry 5 illustrates the preference for condensation at the more-
substituted position under acidic conditions.
7.7.3. Control of Regiochemistry and Stereochemistry of Aldol Reactions of
Ketones
The wide synthetic applicability of the aldol reaction depends on the ability to
achieve both versatility in reactants and control of regiochemistry and stereochemistry.
The term directed aldol addition 96 is applied to reaction conditions that are designed
to achieve specific regio- and stereochemical outcomes. Control of product structure
requires that one reactant act exclusively as the electrophile and the other exclusively
Scheme 7.4. Mixed Aldol Condensation Reactions of Ketones and Aromatic Aldehydes
O O
1 a NaOH
(CH ) CCCH 3 + PhCH O (CH ) CCCH CHPh
3 3
3 3
EtOH-H O 90%
2
O O
KOH
2 b CCH 3 + PhCH O CCH CHPh
72%
O O
3 c CH 3 CH 3
NaOCH 3 O
+
O CH O 75%
CH 3
4 d CH O O
NaOC H
+ CH CH CCH CH 3 2 5 O
2
2
3
CH O
CH 3 60%
O O
HCl
5 e CH CCH CH 3 + PhCH O PhCH CCCH 3
2
3
85%
CH 3
a. G. A. Hill and G. Bramann, Org. Synth., I, 81 (1941).
b. S. C. Bunce, H. J. Dorsman, and F. D. Popp, J. Chem. Soc., 303 (1963).
c. A. M. Islam and M. T. Zenaity, J. Am. Chem. Soc., 79, 6023 (1957).
d. D. Meuche, H. Strauss, and E. Heilbronner, Helv. Chim. Acta, 41, 2220 (1958).
e. M. E. Kronenberg and E. Havinga, Recl. Trav. Chim. Pays-Bas, 84, 17 (1965).
96
T. Mukaiyama, Org. React., 28, 203 (1982).