Page 725 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 725
707
(c) (d) PROBLEMS
1.5
k obs × 10 4 (sec –1 ) 1.0 2 0
0.5
0123456 789 1011 log k obs /S –1 –2
pH
–4
–6
0 2 4 6 8 10
pH
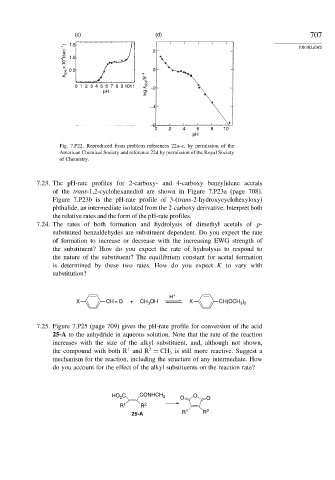
Fig. 7.P22. Reproduced from problem references 22a–c, by permission of the
American Chemical Society and reference 22d by permission of the Royal Society
of Chemistry.
7.23. The pH-rate profiles for 2-carboxy- and 4-carboxy benzylidene acetals
of the trans-1,2-cyclohexanediol are shown in Figure 7.P23a (page 708).
Figure 7.P23b is the pH-rate profile of 3-(trans-2-hydroxycyclohexyloxy)
phthalide, an intermediate isolated from the 2-carboxy derivative. Interpret both
the relative rates and the form of the pH-rate profiles.
7.24. The rates of both formation and hydrolysis of dimethyl acetals of p-
substituted benzaldehydes are substituent dependent. Do you expect the rate
of formation to increase or decrease with the increasing EWG strength of
the substituent? How do you expect the rate of hydrolysis to respond to
the nature of the substituent? The equilibrium constant for acetal formation
is determined by these two rates. How do you expect K to vary with
substitution?
H +
X CH = O + CH OH X CH(OCH )
3 2
3
7.25. Figure 7.P25 (page 709) gives the pH-rate profile for conversion of the acid
25-A to the anhydride in aqueous solution. Note that the rate of the reaction
increases with the size of the alkyl substituent, and, although not shown,
2
1
the compound with both R and R = CH is still more reactive. Suggest a
3
mechanism for the reaction, including the structure of any intermediate. How
do you account for the effect of the alkyl substituents on the reaction rate?
HO C CONHCH 3 O O O
2
R 1 R 2
25-A R 1 R 2