Page 1025 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1025
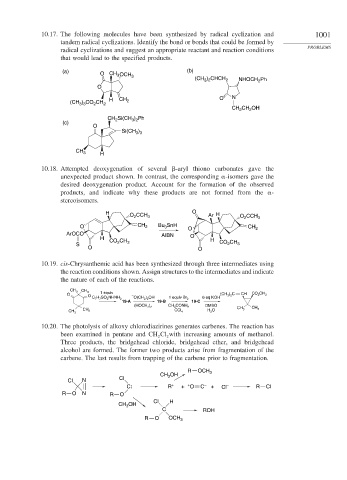
10.17. The following molecules have been synthesized by radical cyclization and 1001
tandem radical cyclizations. Identify the bond or bonds that could be formed by
PROBLEMS
radical cyclizations and suggest an appropriate reactant and reaction conditions
that would lead to the specified products.
(a) O CH 3 OCH (b)
3
(CH ) CHCH 2 NHOCH Ph
3 2
2
O
H CH 2 O N
) CO CH
(CH 3 3 2 2
CH 2 CH 2 OH
CH Si(CH ) Ph
(c) 2 3 2
O
Si(CH )
3 3
CH 3
H
10.18. Attempted deoxygenation of several -aryl thiono carbonates gave the
unexpected product shown. In contrast, the corresponding -isomers gave the
desired deoxygenation product. Account for the formation of the observed
products, and indicate why these products are not formed from the -
stereoisomers.
H O CCH 3 O Ar H O CCH 3
2
2
O CH 2 Bu SnH
3
O CH 2
ArOCO AIBN
H O H
CO 2 CH 3 CO CH
S 2 3
O O
10.19. cis-Chrysanthemic acid has been synthesized through three intermediates using
the reaction conditions shown. Assign structures to the intermediates and indicate
the nature of each of the reactions.
CH 3 CH 3
O O 1 equiv – (CH 3 ) 2 C CH CO 2 CH 3
C 7 H 7 SO 2 NHNH 2 O(CH 2 ) 2 OH 1 equiv Br 2 6 eq KOH
19-A 19-B 19-C
DMSO
(HOCH 2 ) 2 CH 3 CONH 2
CH 3 H 2 O CH 3 CH 3
CH 3 CCl 4
10.20. The photolysis of alkoxy chlorodiazirines generates carbenes. The reaction has
been examined in pentane and CH Cl with increasing amounts of methanol.
2 2
Three products, the bridgehead chloride, bridgehead ether, and bridgehead
alcohol are formed. The former two products arise from fragmentation of the
carbene. The last results from trapping of the carbene prior to fragmentation.
R OCH 3
CH OH
3
Cl N Cl
C: R + + + O C – + Cl – R Cl
R O N R O
Cl H
CH OH
3
C ROH
R O OCH 3