Page 1023 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1023
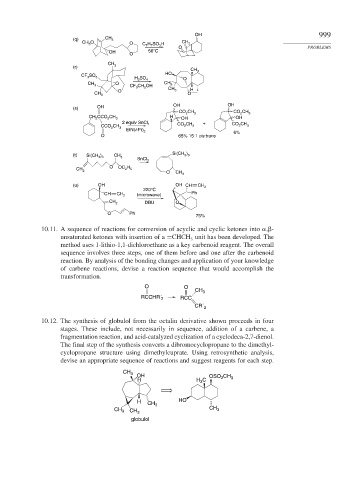
OH 999
(q) CH 3
CH 3 O O C 7 H 7 SO 3 H CH 2
O PROBLEMS
OH 56°C
O
CH 3
(r)
CH 3
HO
CF 3 SO 3
O
H 2 SO 4
O
CH 3 CH 3
CF 3 CH 2 OH
O CH 3 H
CH 3 O
OH OH
(s) OH
CO 2 CH 3 CO 2 CH 3
H
CH 2 CCO 2 CH 3 OH OH
2 equiv SnCl 4 +
CO 2 CH 3 CO 2 CH 3
CCO 2 CH 3
EtN(i-Pr) 2
6%
O 65% 15:1 cis:trans
(t) Si(CH 3 ) 3 CH 3 Si(CH 3 ) 3
SnCl 2
O OC 2 H 5
CH 2
O CH 3
(u) OH OH CH CH 2
220°C
CH CH 2 (microwave) Ph
CH 2 DBU O
O Ph
75%
10.11. A sequence of reactions for conversion of acyclic and cyclic ketones into , -
unsaturated ketones with insertion of a =CHCH unit has been developed. The
3
method uses 1-lithio-1,1-dichloroethane as a key carbenoid reagent. The overall
sequence involves three steps, one of them before and one after the carbenoid
reaction. By analysis of the bonding changes and application of your knowledge
of carbene reactions, devise a reaction sequence that would accomplish the
transformation.
O O
CH 3
RCCHR´ 2 RCC
CR´ 2
10.12. The synthesis of globulol from the octalin derivative shown proceeds in four
stages. These include, not necessarily in sequence, addition of a carbene, a
fragmentation reaction, and acid-catalyzed cyclization of a cyclodeca-2,7-dienol.
The final step of the synthesis converts a dibromocyclopropane to the dimethyl-
cyclopropane structure using dimethylcuprate. Using retrosynthetic analysis,
devise an appropriate sequence of reactions and suggest reagents for each step.
CH 3
OH OSO 2 CH 3
H H C
3
H CH 3 HO
CH 3 CH 3 CH 3
globulol