Page 158 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 158
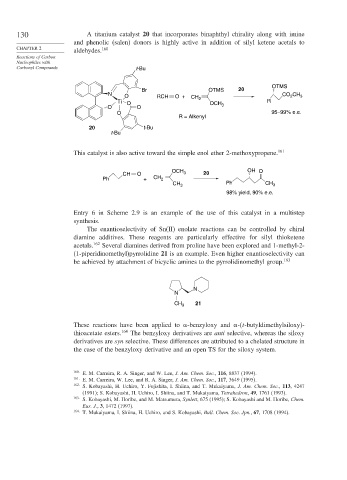
130 A titanium catalyst 20 that incorporates binaphthyl chirality along with imine
and phenolic (salen) donors is highly active in addition of silyl ketene acetals to
CHAPTER 2 160
aldehydes.
Reactions of Carbon
Nucleophiles with
Carbonyl Compounds t-Bu
OTMS
Br OTMS 20
N O RCH O + CO CH 3
2
Ti O CH 2 OCH R
O O 3
O 95–99% e.e.
R = Alkenyl
20 t-Bu
t-Bu
This catalyst is also active toward the simple enol ether 2-methoxypropene. 161
OCH OH O
CH O 3 20
Ph + CH 2
CH 3 Ph CH 3
98% yield, 90% e.e.
Entry 6 in Scheme 2.9 is an example of the use of this catalyst in a multistep
synthesis.
The enantioselectivity of Sn(II) enolate reactions can be controlled by chiral
diamine additives. These reagents are particularly effective for silyl thioketene
acetals. 162 Several diamines derived from proline have been explored and 1-methyl-2-
(1-piperidinomethyl)pyrrolidine 21 is an example. Even higher enantioselectivity can
be achieved by attachment of bicyclic amines to the pyrrolidinomethyl group. 163
N
N
CH 3 21
These reactions have been applied to -benzyloxy and -(t-butyldimethylsiloxy)-
thioacetate esters. 164 The benzyloxy derivatives are anti selective, whereas the siloxy
derivatives are syn selective. These differences are attributed to a chelated structure in
the case of the benzyloxy derivative and an open TS for the siloxy system.
160 E. M. Carreira, R. A. Singer, and W. Lee, J. Am. Chem. Soc., 116, 8837 (1994).
161
E. M. Carreira, W. Lee, and R. A. Singer, J. Am. Chem. Soc., 117, 3649 (1995).
162 S. Kobayashi, H. Uchiro, Y. Fujishita, I. Shiina, and T. Mukaiyama, J. Am. Chem. Soc., 113, 4247
(1991); S. Kobayashi, H. Uchiro, I. Shiina, and T. Mukaiyama, Tetrahedron, 49, 1761 (1993).
163 S. Kobayashi, M. Horibe, and M. Matsumura, Synlett, 675 (1995); S. Kobayashi and M. Horibe, Chem.
Eur. J., 3, 1472 (1997).
164
T. Mukaiyama, I. Shiina, H. Uchiro, and S. Kobayashi, Bull. Chem. Soc. Jpn., 67, 1708 (1994).