Page 155 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 155
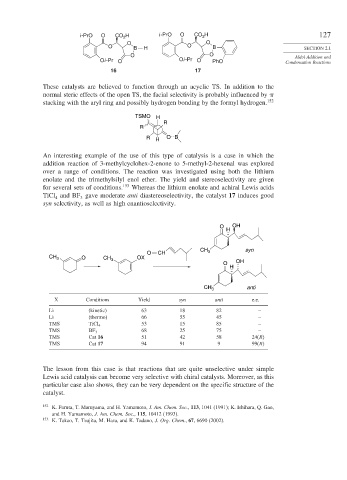
i -PrO O CO H i -PrO O CO H 127
2
2
O O
O B H O B SECTION 2.1
O O Aldol Addition and
Oi -Pr O Oi -Pr O PhO Condensation Reactions
16 17
These catalysts are believed to function through an acyclic TS. In addition to the
normal steric effects of the open TS, the facial selectivity is probably influenced by
stacking with the aryl ring and possibly hydrogen bonding by the formyl hydrogen. 152
TSMO H
R
R
R H OB
An interesting example of the use of this type of catalysis is a case in which the
addition reaction of 3-methylcyclohex-2-enone to 5-methyl-2-hexenal was explored
over a range of conditions. The reaction was investigated using both the lithium
enolate and the trimethylsilyl enol ether. The yield and stereoselectivity are given
for several sets of conditions. 153 Whereas the lithium enolate and achiral Lewis acids
TiCl and BF gave moderate anti diastereoselectivity, the catalyst 17 induces good
4 3
syn selectivity, as well as high enantioselectivity.
O OH
H
CH syn
O CH 3
CH 3 O CH 3 OX
O OH
H
CH 3 anti
X Conditions Yield syn anti e.e.
Li (kinetic) 63 18 82 –
Li (thermo) 66 55 45 –
TMS TiCl 4 53 15 85 –
TMS BF 3 68 25 75 –
TMS Cat 16 51 42 58 24(R)
TMS Cat 17 94 91 9 99(R)
The lesson from this case is that reactions that are quite unselective under simple
Lewis acid catalysis can become very selective with chiral catalysts. Moreover, as this
particular case also shows, they can be very dependent on the specific structure of the
catalyst.
152 K. Furuta, T. Maruyama, and H. Yamamoto, J. Am. Chem. Soc., 113, 1041 (1991); K. Ishihara, Q. Gao,
and H. Yamamoto, J. Am. Chem. Soc., 115, 10412 (1993).
153
K. Takao, T. Tsujita, M. Hara, and K. Tadano, J. Org. Chem., 67, 6690 (2002).