Page 152 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 152
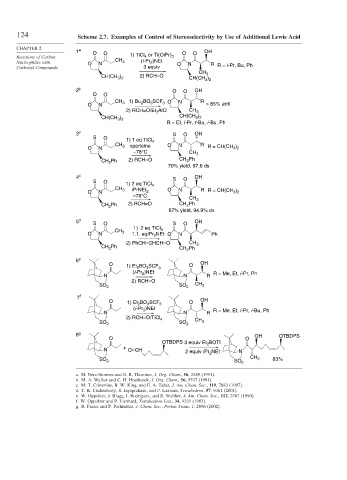
124 Scheme 2.7. Examples of Control of Stereoselectivity by Use of Additional Lewis Acid
CHAPTER 2
1 a O O O O OH
Reactions of Carbon 1) TiCl 4 or Ti(OiPr) 3
Nucleophiles with O N CH 3 (i-Pr 2 )NEt O N R R = i-Pr, Bu, Ph
Carbonyl Compounds 3 equiv
CH 3
2) RCH=O
CH(CH 3 ) 2
CH(CH 3 ) 2
2 b O O OH
O O
CH 3 1) Bu 2 BO 3 SCF 3 O N R
O N > 85% anti
2) RCH=O/Et 2 AlCl CH 3
CH(CH 3 ) 2
CH(CH 3 ) 2
R = Et, i -Pr, t -Bu, i -Bu, Ph
3 c S O OH
S O
1) 1 eq TiCl 4
CH 3 sparteine O N R
O N R = CH(CH 3 ) 2
–78°C CH 3
CH 2 Ph 2) RCH=O CH 2 Ph
70% yield, 97.6 ds
4 c S O OH
S O
1) 2 eq TiCl 4
CH 3 O N
O N i PrNEt 2 R R = CH(CH 3 ) 2
–78°C
CH 3
CH 2 Ph 2) RCH=O CH 2 Ph
87% yield, 94.9% ds
5 d S O S O OH
1) 2 eq TiCl 4
CH 3
O N 1.1. eqi Pr 2 NEt O N Ph
2) PhCH=CHCH=O CH 3
CH 2 Ph CH 2 Ph
6 e
O O OH
1) Et 2 BO 3 SCF 3
(i-Pr 2 )NEt
N N R R = Me, Et, i -Pr, Ph
2) RCH=O
CH 3
SO 2 SO 2
7 f
O O OH
1) Et 2 BO 3 SCF 3
(i -Pr 2 )NEt R = Me, Et, i -Pr, i -Bu, Ph
N N R
2) RCH=O/TiCl 4
CH 3
SO 2 SO 2
8 g OH OTBDPS
O O
OTBDPS 3 equiv Et 2 BOTf
+
N O=CH 2 equiv i Pr 2 NEt N
CH 3 83%
SO 2
SO 2
a. M. Nerz-Stormes and E. R. Thornton, J. Org. Chem., 56, 2489 (1991).
b. M. A. Walker and C. H. Heathcock, J. Org. Chem., 56, 5747 (1991).
c. M. T. Crimmins, B. W. King, and E. A. Tabet, J. Am. Chem. Soc., 119, 7883 (1997).
d. T. K. Chakraborty, S. Jayaprakash, and P. Laxman, Tetrahedron, 57, 9461 (2001).
e. W. Oppolzer, J. Blagg, I. Rodriguez, and E. Walther, J. Am. Chem. Soc., 112, 2767 (1990).
f. W. Oppolzer and P. Lienhard, Tetrahedron Lett., 34, 4321 (1993).
g. B. Fraser and P. Perlmutter, J. Chem. Soc., Perkin Trans. 1, 2896 (2002).